 Purple glow in its plasma state | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | colorless gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(H) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | 1s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | (H2) 13.99 K (−259.16 °C, −434.49 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | (H2) 20.271 K (−252.879 °C, −423.182 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at STP) | 0.08988 g/L | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 0.07 g/cm3 (solid: 0.0763 g/cm3)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at b.p.) | 0.07099 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 13.8033 K, 7.041 kPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 32.938 K, 1.2858 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | (H2) 0.117 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | (H2) 0.904 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | (H2) 28.836 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, +1 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 31±5 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 120 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of hydrogen | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 1310 m/s (gas, 27 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.1805 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −3.98×10−6 cm3/mol (298 K)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 12385-13-6 1333-74-0 (H2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Henry Cavendish[5][6] (1766) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Named by | Antoine Lavoisier[7] (1783) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of hydrogen
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H2. It is colorless, odorless, tasteless,[8] non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.[9][note 1] Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons.
In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 years later during the recombination epoch, when the plasma had cooled enough for electrons to remain bound to protons.[10]
Hydrogen is nonmetallic (except it becomes metallic at extremely high pressures) and readily forms a single covalent bond with most nonmetallic elements, forming compounds such as water and nearly all organic compounds. Hydrogen plays a particularly important role in acid–base reactions because these reactions usually involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) where it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The H+ cation is simply a proton (symbol p) but its behavior in aqueous solutions and in ionic compounds involves screening of its electric charge by nearby polar molecules or anions. Because hydrogen is the only neutral atom for which the Schrödinger equation can be solved analytically,[11] the study of its energetics and chemical bonding has played a key role in the development of quantum mechanics.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–1781, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance,[12] and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means «water-former».
Industrial production is mainly from steam reforming of natural gas, oil reforming, or coal gasification.[13] A small percentage is also produced using more energy-intensive methods such as the electrolysis of water.[13][14][15] Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. It can be burned to produce heat or combined with oxygen in fuel cells to generate electricity directly, with water being the only emissions at the point of usage. Hydrogen atoms (but not gaseous molecules) are problematic in metallurgy because they can embrittle many metals.[16]
Properties
Combustion
Combustion of hydrogen with the oxygen in the air. When the bottom cap is removed, allowing air to enter at the bottom, the hydrogen in the container rises out of top and burns as it mixes with the air.

Hydrogen gas (dihydrogen or molecular hydrogen)[17] is highly flammable:
- 2 H2(g) + O2(g) → 2 H2O(l) (572 kJ/2 mol = 286 kJ/mol = 141.865 MJ/kg)[note 2]
The enthalpy of combustion is −286 kJ/mol.[18]
Hydrogen gas forms explosive mixtures with air in concentrations from 4–74%[19] and with chlorine at 5–95%. The explosive reactions may be triggered by spark, heat, or sunlight. The hydrogen autoignition temperature, the temperature of spontaneous ignition in air, is 500 °C (932 °F).[20]
Flame
Pure hydrogen-oxygen flames emit ultraviolet light and with high oxygen mix are nearly invisible to the naked eye, as illustrated by the faint plume of the Space Shuttle Main Engine, compared to the highly visible plume of a Space Shuttle Solid Rocket Booster, which uses an ammonium perchlorate composite. The detection of a burning hydrogen leak may require a flame detector; such leaks can be very dangerous. Hydrogen flames in other conditions are blue, resembling blue natural gas flames.[21] The destruction of the Hindenburg airship was a notorious example of hydrogen combustion and the cause is still debated. The visible flames in the photographs were the result of carbon compounds in the airship skin burning.[22]
Reactants
H2 is unreactive compared to diatomic elements such as halogens or oxygen. The thermodynamic basis of this low reactivity is the very strong H–H bond, with a bond dissociation energy of 435.7 kJ/mol.[23] The kinetic basis of the low reactivity is the nonpolar nature of H2 and its weak polarizability. It spontaneously reacts with chlorine and fluorine to form hydrogen chloride and hydrogen fluoride, respectively.[24] The reactivity of H2 is strongly affected by the presence of metal catalysts. Thus, while mixtures of H2 with O2 or air combust readily when heated to at least 500 °C by a spark or flame, they do not react at room temperature in the absence of a catalyst.
Electron energy levels

Depiction of a hydrogen atom with size of central proton shown, and the atomic diameter shown as about twice the Bohr model radius (image not to scale)
The ground state energy level of the electron in a hydrogen atom is −13.6 eV,[25] which is equivalent to an ultraviolet photon of roughly 91 nm wavelength.[26]
The energy levels of hydrogen can be calculated fairly accurately using the Bohr model of the atom, which conceptualizes the electron as «orbiting» the proton in analogy to the Earth’s orbit of the Sun. However, the atomic electron and proton are held together by electromagnetic force, while planets and celestial objects are held by gravity. Because of the discretization of angular momentum postulated in early quantum mechanics by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.[27]
A more accurate description of the hydrogen atom comes from a purely quantum mechanical treatment that uses the Schrödinger equation, Dirac equation or Feynman path integral formulation to calculate the probability density of the electron around the proton.[28] The most complicated treatments allow for the small effects of special relativity and vacuum polarization. In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum at all—illustrating how the «planetary orbit» differs from electron motion.
Spin isomers
Molecular H2 exists as two spin isomers, i.e. compounds that differ only in the spin states of their nuclei.[29] In the orthohydrogen form, the spins of the two nuclei are parallel, forming a spin triplet state having a total molecular spin 

The ortho-to-para ratio in H2 is an important consideration in the liquefaction and storage of liquid hydrogen: the conversion from ortho to para is exothermic and produces enough heat to evaporate a most of the liquid if not converted first to parahydrogen during the cooling process.[34] Catalysts for the ortho-para interconversion, such as ferric oxide and activated carbon compounds, are used during hydrogen cooling to avoid this loss of liquid.[35]
Phases

Hydrogen gas is colorless and transparent, here contained in a glass ampoule.

- Gaseous hydrogen
- Liquid hydrogen
- Slush hydrogen
- Solid hydrogen
- Metallic hydrogen
- Plasma hydrogen
Compounds
Covalent and organic compounds
While H2 is not very reactive under standard conditions, it does form compounds with most elements. Hydrogen can form compounds with elements that are more electronegative, such as halogens (F, Cl, Br, I), or oxygen; in these compounds hydrogen takes on a partial positive charge.[36] When bonded to a more electronegative element, particularly fluorine, oxygen, or nitrogen, hydrogen can participate in a form of medium-strength noncovalent bonding with another electronegative element with a lone pair, a phenomenon called hydrogen bonding that is critical to the stability of many biological molecules.[37][38] Hydrogen also forms compounds with less electronegative elements, such as metals and metalloids, where it takes on a partial negative charge. These compounds are often known as hydrides.[39]
Hydrogen forms a vast array of compounds with carbon called the hydrocarbons, and an even vaster array with heteroatoms that, because of their general association with living things, are called organic compounds.[40] The study of their properties is known as organic chemistry[41] and their study in the context of living organisms is known as biochemistry.[42] By some definitions, «organic» compounds are only required to contain carbon. However, most of them also contain hydrogen, and because it is the carbon-hydrogen bond that gives this class of compounds most of its particular chemical characteristics, carbon-hydrogen bonds are required in some definitions of the word «organic» in chemistry.[40] Millions of hydrocarbons are known, and they are usually formed by complicated pathways that seldom involve elemental hydrogen.
Hydrogen is highly soluble in many rare earth and transition metals[43] and is soluble in both nanocrystalline and amorphous metals.[44] Hydrogen solubility in metals is influenced by local distortions or impurities in the crystal lattice.[45] These properties may be useful when hydrogen is purified by passage through hot palladium disks, but the gas’s high solubility is a metallurgical problem, contributing to the embrittlement of many metals,[16] complicating the design of pipelines and storage tanks.[46]
Hydrides

Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. The term «hydride» suggests that the H atom has acquired a negative or anionic character, denoted H−, and is used when hydrogen forms a compound with a more electropositive element. The existence of the hydride anion, suggested by Gilbert N. Lewis in 1916 for group 1 and 2 salt-like hydrides, was demonstrated by Moers in 1920 by the electrolysis of molten lithium hydride (LiH), producing a stoichiometric quantity of hydrogen at the anode.[47] For hydrides other than group 1 and 2 metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group 2 hydrides is BeH2, which is polymeric. In lithium aluminium hydride, the [AlH4]− anion carries hydridic centers firmly attached to the Al(III).
Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, more than 100 binary borane hydrides are known, but only one binary aluminium hydride.[48] Binary indium hydride has not yet been identified, although larger complexes exist.[49]
In inorganic chemistry, hydrides can also serve as bridging ligands that link two metal centers in a coordination complex. This function is particularly common in group 13 elements, especially in boranes (boron hydrides) and aluminium complexes, as well as in clustered carboranes.[50]
Protons and acids
Oxidation of hydrogen removes its electron and gives H+, which contains no electrons and a nucleus which is usually composed of one proton. That is why H+ is often called a proton. This species is central to discussion of acids. Under the Brønsted–Lowry acid–base theory, acids are proton donors, while bases are proton acceptors.
A bare proton, H+, cannot exist in solution or in ionic crystals because of its unstoppable attraction to other atoms or molecules with electrons. Except at the high temperatures associated with plasmas, such protons cannot be removed from the electron clouds of atoms and molecules, and will remain attached to them. However, the term ‘proton’ is sometimes used loosely and metaphorically to refer to positively charged or cationic hydrogen attached to other species in this fashion, and as such is denoted «H+» without any implication that any single protons exist freely as a species.
To avoid the implication of the naked «solvated proton» in solution, acidic aqueous solutions are sometimes considered to contain a less unlikely fictitious species, termed the «hydronium ion» ([H3O]+). However, even in this case, such solvated hydrogen cations are more realistically conceived as being organized into clusters that form species closer to [H9O4]+.[51] Other oxonium ions are found when water is in acidic solution with other solvents.[52]
Although exotic on Earth, one of the most common ions in the universe is the H+3 ion, known as protonated molecular hydrogen or the trihydrogen cation.[53]
Isotopes


Hydrogen discharge (spectrum) tube

Deuterium discharge (spectrum) tube
Hydrogen has three naturally occurring isotopes, denoted 1
H, 2
H and 3
H. Other, highly unstable nuclei (4
H to 7
H) have been synthesized in the laboratory but not observed in nature.[54][55]
- 1
H is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name protium.[56] It is unique among all stable isotopes in having no neutrons; see diproton for a discussion of why others do not exist. - 2
H, the other stable hydrogen isotope, is known as deuterium and contains one proton and one neutron in the nucleus. All deuterium in the universe is thought to have been produced at the time of the Big Bang, and has endured since that time. Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called heavy water. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for 1
H-NMR spectroscopy.[57] Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion.[58] - 3
H is known as tritium and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into helium-3 through beta decay with a half-life of 12.32 years.[50] It is so radioactive that it can be used in luminous paint, making it useful in such things as watches. The glass prevents the small amount of radiation from getting out.[59] Small amounts of tritium are produced naturally by the interaction of cosmic rays with atmospheric gases; tritium has also been released during nuclear weapons tests.[60] It is used in nuclear fusion reactions,[61] as a tracer in isotope geochemistry,[62] and in specialized self-powered lighting devices.[63] Tritium has also been used in chemical and biological labeling experiments as a radiolabel.[64]
Unique among the elements, distinct names are assigned to its isotopes in common use today. During the early study of radioactivity, various heavy radioactive isotopes were given their own names, but such names are no longer used, except for deuterium and tritium. The symbols D and T (instead of 2
H and 3
H) are sometimes used for deuterium and tritium, but the symbol P is already in use for phosphorus and thus is not available for protium.[65] In its nomenclatural guidelines, the International Union of Pure and Applied Chemistry (IUPAC) allows any of D, T, 2
H, and 3
H to be used, although 2
H and 3
H are preferred.[66]
The exotic atom muonium (symbol Mu), composed of an antimuon and an electron, can also be considered a light radioisotope of hydrogen.[67] Because muons decay with lifetime 2.2 µs, muonium is too unstable to exhibit observable chemistry.[68] Nevertheless, muonium compounds are important test cases for quantum simulation, due to the mass difference between the antimuon and the proton,[69] and IUPAC nomenclature incorporates such hypothetical compounds as muonium chloride (MuCl) and sodium muonide (NaMu), analogous to hydrogen chloride and sodium hydride respectively.[70]
Thermal and physical properties
Table of thermal and physical properties of hydrogen (H2) at atmospheric pressure:[71][72]
| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg °C) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Thermal conductivity (W/m °C) | Thermal diffusivity (m^2/s) | Prandtl Number |
| 100 | 0.24255 | 11.23 | 4.21E-06 | 1.74E-05 | 6.70E-02 | 2.46E-05 | 0.707 |
| 150 | 0.16371 | 12.602 | 5.60E-06 | 3.42E-05 | 0.0981 | 4.75E-05 | 0.718 |
| 200 | 0.1227 | 13.54 | 6.81E-06 | 5.55E-05 | 0.1282 | 7.72E-05 | 0.719 |
| 250 | 0.09819 | 14.059 | 7.92E-06 | 8.06E-05 | 0.1561 | 1.13E-04 | 0.713 |
| 300 | 0.08185 | 14.314 | 8.96E-06 | 1.10E-04 | 0.182 | 1.55E-04 | 0.706 |
| 350 | 0.07016 | 14.436 | 9.95E-06 | 1.42E-04 | 0.206 | 2.03E-04 | 0.697 |
| 400 | 0.06135 | 14.491 | 1.09E-05 | 1.77E-04 | 0.228 | 2.57E-04 | 0.69 |
| 450 | 0.05462 | 14.499 | 1.18E-05 | 2.16E-04 | 0.251 | 3.16E-04 | 0.682 |
| 500 | 0.04918 | 14.507 | 1.26E-05 | 2.57E-04 | 0.272 | 3.82E-04 | 0.675 |
| 550 | 0.04469 | 14.532 | 1.35E-05 | 3.02E-04 | 0.292 | 4.52E-04 | 0.668 |
| 600 | 0.04085 | 14.537 | 1.43E-05 | 3.50E-04 | 0.315 | 5.31E-04 | 0.664 |
| 700 | 0.03492 | 14.574 | 1.59E-05 | 4.55E-04 | 0.351 | 6.90E-04 | 0.659 |
| 800 | 0.0306 | 14.675 | 1.74E-05 | 5.69E-04 | 0.384 | 8.56E-04 | 0.664 |
| 900 | 0.02723 | 14.821 | 1.88E-05 | 6.90E-04 | 0.412 | 1.02E-03 | 0.676 |
| 1000 | 0.02424 | 14.99 | 2.01E-05 | 8.30E-04 | 0.448 | 1.23E-03 | 0.673 |
| 1100 | 0.02204 | 15.17 | 2.13E-05 | 9.66E-04 | 0.488 | 1.46E-03 | 0.662 |
| 1200 | 0.0202 | 15.37 | 2.26E-05 | 1.12E-03 | 0.528 | 1.70E-03 | 0.659 |
| 1300 | 0.01865 | 15.59 | 2.39E-05 | 1.28E-03 | 0.568 | 1.96E-03 | 0.655 |
| 1400 | 0.01732 | 15.81 | 2.51E-05 | 1.45E-03 | 0.61 | 2.23E-03 | 0.65 |
| 1500 | 0.01616 | 16.02 | 2.63E-05 | 1.63E-03 | 0.655 | 2.53E-03 | 0.643 |
| 1600 | 0.0152 | 16.28 | 2.74E-05 | 1.80E-03 | 0.697 | 2.82E-03 | 0.639 |
| 1700 | 0.0143 | 16.58 | 2.85E-05 | 1.99E-03 | 0.742 | 3.13E-03 | 0.637 |
| 1800 | 0.0135 | 16.96 | 2.96E-05 | 2.19E-03 | 0.786 | 3.44E-03 | 0.639 |
| 1900 | 0.0128 | 17.49 | 3.07E-05 | 2.40E-03 | 0.835 | 3.73E-03 | 0.643 |
| 2000 | 0.0121 | 18.25 | 3.18E-05 | 2.63E-03 | 0.878 | 3.98E-03 | 0.661 |
History
Discovery and use
In 1671, Robert Boyle discovered and described the reaction between iron filings and dilute acids, which results in the production of hydrogen gas.[73][74]
Having provided a saline spirit [hydrochloric acid], which by an uncommon way of preparation was made exceeding sharp and piercing, we put into a vial, capable of containing three or four ounces of water, a convenient quantity of filings of steel, which were not such as are commonly sold in shops to Chymists and Apothecaries, (those being usually not free enough from rust) but such as I had a while before caus’d to be purposely fil’d off from a piece of good steel. This metalline powder being moistn’d in the viol with a little of the menstruum, was afterwards drench’d with more; whereupon the mixture grew very hot, and belch’d up copious and stinking fumes; which whether they consisted altogether of the volatile sulphur of the Mars [iron?], or of metalline steams participating of a sulphureous nature, and join’d with the saline exhalations of the menstruum, is not necessary to be here discuss’d. But whencesoever this stinking smoak proceeded, so inflammable it was, that upon the approach of a lighted candle to it, it would readily enough take fire, and burn with a blewish and somewhat greenish flame at the mouth of the viol for a good while together; and that, though with little light, yet with more strength than one would easily suspect.
— Robert Boyle, Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air…
In 1766, Henry Cavendish was the first to recognize hydrogen gas as a discrete substance, by naming the gas from a metal-acid reaction «inflammable air». He speculated that «inflammable air» was in fact identical to the hypothetical substance called «phlogiston»[75][76] and further finding in 1781 that the gas produces water when burned. He is usually given credit for the discovery of hydrogen as an element.[5][6] In 1783, Antoine Lavoisier gave the element the name hydrogen (from the Greek ὑδρο- hydro meaning «water» and -γενής genes meaning «former»)[77] when he and Laplace reproduced Cavendish’s finding that water is produced when hydrogen is burned.[6]

Antoine-Laurent de Lavoisier
Lavoisier produced hydrogen for his experiments on mass conservation by reacting a flux of steam with metallic iron through an incandescent iron tube heated in a fire. Anaerobic oxidation of iron by the protons of water at high temperature can be schematically represented by the set of following reactions:
- 1) Fe + H2O → FeO + H2
- 2) Fe + 3 H2O → Fe2O3 + 3 H2
- 3) Fe + 4 H2O → Fe3O4 + 4 H2
Many metals such as zirconium undergo a similar reaction with water leading to the production of hydrogen.
Hydrogen was liquefied for the first time by James Dewar in 1898 by using regenerative cooling and his invention, the vacuum flask.[6] He produced solid hydrogen the next year.[6] Deuterium was discovered in December 1931 by Harold Urey, and tritium was prepared in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck.[5] Heavy water, which consists of deuterium in the place of regular hydrogen, was discovered by Urey’s group in 1932.[6] François Isaac de Rivaz built the first de Rivaz engine, an internal combustion engine powered by a mixture of hydrogen and oxygen in 1806. Edward Daniel Clarke invented the hydrogen gas blowpipe in 1819. The Döbereiner’s lamp and limelight were invented in 1823.[6]
The first hydrogen-filled balloon was invented by Jacques Charles in 1783.[6] Hydrogen provided the lift for the first reliable form of air-travel following the 1852 invention of the first hydrogen-lifted airship by Henri Giffard.[6] German count Ferdinand von Zeppelin promoted the idea of rigid airships lifted by hydrogen that later were called Zeppelins; the first of which had its maiden flight in 1900.[6] Regularly scheduled flights started in 1910 and by the outbreak of World War I in August 1914, they had carried 35,000 passengers without a serious incident. Hydrogen-lifted airships were used as observation platforms and bombers during the war.
The first non-stop transatlantic crossing was made by the British airship R34 in 1919. Regular passenger service resumed in the 1920s and the discovery of helium reserves in the United States promised increased safety, but the U.S. government refused to sell the gas for this purpose. Therefore, H2 was used in the Hindenburg airship, which was destroyed in a midair fire over New Jersey on 6 May 1937.[6] The incident was broadcast live on radio and filmed. Ignition of leaking hydrogen is widely assumed to be the cause, but later investigations pointed to the ignition of the aluminized fabric coating by static electricity. But the damage to hydrogen’s reputation as a lifting gas was already done and commercial hydrogen airship travel ceased. Hydrogen is still used, in preference to non-flammable but more expensive helium, as a lifting gas for weather balloons.
In the same year, the first hydrogen-cooled turbogenerator went into service with gaseous hydrogen as a coolant in the rotor and the stator in 1937 at Dayton, Ohio, by the Dayton Power & Light Co.;[78] because of the thermal conductivity and very low viscosity of hydrogen gas, thus lower drag than air, this is the most common type in its field today for large generators (typically 60 MW and bigger; smaller generators are usually air-cooled).
The nickel hydrogen battery was used for the first time in 1977 aboard the U.S. Navy’s Navigation technology satellite-2 (NTS-2).[79] For example, the ISS,[80] Mars Odyssey[81] and the Mars Global Surveyor[82] are equipped with nickel-hydrogen batteries. In the dark part of its orbit, the Hubble Space Telescope is also powered by nickel-hydrogen batteries, which were finally replaced in May 2009,[83] more than 19 years after launch and 13 years beyond their design life.[84]
Role in quantum theory

Hydrogen emission spectrum lines in the visible range. These are the four visible lines of the Balmer series
Because of its simple atomic structure, consisting only of a proton and an electron, the hydrogen atom, together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of atomic structure.[85] Furthermore, study of the corresponding simplicity of the hydrogen molecule and the corresponding cation H+2 brought understanding of the nature of the chemical bond, which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s.
One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full quantum mechanical theory arrived. Maxwell observed that the specific heat capacity of H2 unaccountably departs from that of a diatomic gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in H2 because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.[86]
Antihydrogen (
H
) is the antimatter counterpart to hydrogen. It consists of an antiproton with a positron. Antihydrogen is the only type of antimatter atom to have been produced as of 2015.[87][88]
Cosmic prevalence and distribution

Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.[89]) This element is found in great abundance in stars and gas giant planets. Molecular clouds of H2 are associated with star formation. Hydrogen plays a vital role in powering stars through the proton-proton reaction in case of stars with very low to approximately 1 mass of the Sun and the CNO cycle of nuclear fusion in case of stars more massive than our Sun.[90]
States
Throughout the universe, hydrogen is mostly found in the atomic and plasma states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen’s electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind they interact with the Earth’s magnetosphere giving rise to Birkeland currents and the aurora.
Hydrogen is found in the neutral atomic state in the interstellar medium because the atoms seldom collide and combine. They are the source of the 21-cm hydrogen line at 1420 MHz that is detected in order to probe primordial hydrogen.[91] The large amount of neutral hydrogen found in the damped Lyman-alpha systems is thought to dominate the cosmological baryonic density of the universe up to a redshift of z = 4.[92]
Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, H2. Hydrogen gas is very rare in the Earth’s atmosphere (1 ppm by volume) because of its light weight, which enables it to escape from the atmosphere more rapidly than heavier gases. However, hydrogen is the third most abundant element on the Earth’s surface,[93] mostly in the form of chemical compounds such as hydrocarbons and water.[50]
A molecular form called protonated molecular hydrogen (H+3) is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic rays. This ion has also been observed in the upper atmosphere of the planet Jupiter. The ion is relatively stable in the environment of outer space due to the low temperature and density. H+3 is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium.[94] Neutral triatomic hydrogen H3 can exist only in an excited form and is unstable.[95] By contrast, the positive hydrogen molecular ion (H+2) is a rare molecule in the universe.
Production
H2 is produced in chemistry and biology laboratories, often as a by-product of other reactions; in industry for the hydrogenation of unsaturated substrates; and in nature as a means of expelling reducing equivalents in biochemical reactions.
Water electrolysis

Illustrating inputs and outputs of simple electrolysis of water production of hydrogen
The electrolysis of water is a simple method of producing hydrogen. A current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.[96][97]
- 2 H2O(l) → 2 H2(g) + O2(g)
Methane pyrolysis

Hydrogen production using natural gas methane pyrolysis is a one-step process that produces no greenhouse gases.[98][99][100][101] Developing volume production using this method is the key to enabling faster carbon reduction by using hydrogen in industrial processes,[102] fuel cell electric heavy truck transportation,[103][104][105][106] and in gas turbine electric power generation.[107][108] Methane pyrolysis is performed by having methane CH4 bubbled up through a molten metal catalyst containing dissolved nickel at 1,340 K (1,070 °C; 1,950 °F). This causes the methane to break down into hydrogen gas and solid carbon, with no other byproducts.[109][110]
- CH4(g) → C(s) + 2 H2(g) (ΔH° = 74 kJ/mol)
The industrial quality solid carbon may be sold as manufacturing feedstock or permanently landfilled; it is not released into the atmosphere and does not cause ground water pollution in landfill. Methane pyrolysis is in development and considered suitable for commercial bulk hydrogen production. Volume production is being evaluated in the BASF «methane pyrolysis at scale» pilot plant.[111] Further research continues in several laboratories, including at Karlsruhe Liquid-metal Laboratory (KALLA)[112] and the chemical engineering laboratory at University of California – Santa Barbara[113]
Other industrial methods

Illustrating inputs and outputs of steam reforming of natural gas, a process to produce hydrogen[image reference needed]
Hydrogen is often produced by reacting water with methane and carbon monoxide, which causes the removal of hydrogen from hydrocarbons at very high temperatures, with 48% of hydrogen production coming from steam reforming.[114][115] The water vapor is then reacted with the carbon monoxide produced by steam reforming to oxidize it to carbon dioxide and turn the water into hydrogen. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas[116] with release of atmospheric greenhouse gas or with capture using CCS and climate change mitigation. Steam reforming is also known as the Bosch process and is widely used for the industrial preparation of hydrogen.
At high temperatures (1000–1400 K, 700–1100 °C or 1300–2000 °F), steam (water vapor) reacts with methane to yield carbon monoxide and H2.
- CH4 + H2O → CO + 3 H2
This reaction is favored at low pressures but is nonetheless conducted at high pressures (2.0 MPa, 20 atm or 600 inHg). This is because high-pressure H2 is the most marketable product, and pressure swing adsorption (PSA) purification systems work better at higher pressures. The product mixture is known as «synthesis gas» because it is often used directly for the production of methanol and related compounds. Hydrocarbons other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
- CH4 → C + 2 H2
Consequently, steam reforming typically employs an excess of H2O. Additional hydrogen can be recovered from the steam by use of carbon monoxide through the water gas shift reaction, especially with an iron oxide catalyst. This reaction is also a common industrial source of carbon dioxide:[116]
- CO + H2O → CO2 + H2
Other important methods for CO and H2 production include partial oxidation of hydrocarbons:[117]
- 2 CH4 + O2 → 2 CO + 4 H2
and the coal reaction, which can serve as a prelude to the shift reaction above:[116]
- C + H2O → CO + H2
Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the Haber process for the production of ammonia, hydrogen is generated from natural gas.[118] Electrolysis of brine to yield chlorine also produces hydrogen as a co-product.[119]
Olefin production units may produce substantial quantities of byproduct hydrogen particularly from cracking light feedstocks like ethane or propane.
Metal-acid
Many metals react with water to produce H2, but the rate of hydrogen evolution depends on the metal, the pH, and the presence alloying agents. Most commonly, hydrogen evolution is induced by acids. The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids. This reaction is the basis of the Kipp’s apparatus, which once was used as a laboratory gas source:
- Zn + 2 H+ → Zn2+ + H2
In the absence of acid, the evolution of H2 is slower. Because iron is widely used structural material, its anaerobic corrosion is of technological significance:
- Fe + 2 H2O → Fe(OH)2 + H2
Many metals, such as aluminium, are slow to react with water because they form passivated coatings of oxides. An alloy of aluminium and gallium, however, does react with water.[120] At high pH, aluminium can produce H2:
- 2 Al + 6 H2O + 2 OH− → 2 [Al(OH)4]− + 3 H2
Some metal-containing compounds react with acids to evolve H2. Under anaerobic conditions, ferrous hydroxide (Fe(OH)
2) can be oxidized by the protons of water to form magnetite and H2. This process is described by the Schikorr reaction:
- 3 Fe(OH)2 → Fe3O4 + 2 H2O + H2
This process occurs during the anaerobic corrosion of iron and steel in oxygen-free groundwater and in reducing soils below the water table.
Thermochemical
More than 200 thermochemical cycles can be used for water splitting. Many of these cycles such as the iron oxide cycle, cerium(IV) oxide–cerium(III) oxide cycle, zinc zinc-oxide cycle, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity.[121] A number of laboratories (including in France, Germany, Greece, Japan, and the United States) are developing thermochemical methods to produce hydrogen from solar energy and water.[122]
Serpentinization reaction
In deep geological conditions prevailing far away from the Earth’s atmosphere, hydrogen (H2) is produced during the process of serpentinization. In this process, water protons (H+) are reduced by ferrous (Fe2+) ions provided by fayalite (Fe2SiO4). The reaction forms magnetite (Fe3O4), quartz (SiO2), and hydrogen (H2):[123][124]
- 3 Fe2SiO4 + 2 H2O → 2 Fe3O4 + 3 SiO2 + 3 H2
- fayalite + water → magnetite + quartz + hydrogen
This reaction closely resembles the Schikorr reaction observed in anaerobic oxidation of ferrous hydroxide in contact with water.
Applications
Petrochemical industry
Large quantities of H2 are used in the «upgrading» of fossil fuels. Key consumers of H2 include hydrodealkylation, hydrodesulfurization, and hydrocracking. Many of these reactions can be classified as hydrogenolysis, i.e., the cleavage of bonds to carbon. Illustrative is the separation of sulfur from liquid fossil fuels:
- R2S + 2 H2 → H2S + 2 RH
Hydrogenation
Hydrogenation, the addition of H2 to various substrates is conducted on a large scale. The hydrogenation of N2 to produce ammonia by the Haber–Bosch process consumes a few percent of the energy budget in the entire industry. The resulting ammonia is used to supply the majority of the protein consumed by humans.[125] Hydrogenation is used to convert unsaturated fats and oils to saturated fats and oils. The major application is the production of margarine. Methanol is produced by hydrogenation of carbon dioxide. It is similarly the source of hydrogen in the manufacture of hydrochloric acid. H2 is also used as a reducing agent for the conversion of some ores to the metals.[126]
Coolant
Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include low density, low viscosity, and the highest specific heat and thermal conductivity of all gases.
Energy carrier
Elemental hydrogen has been widely discussed in the context of energy, as a possible future carrier of energy on an economy-wide scale.[127] Hydrogen is a »carrier» of energy rather than an energy resource, because there is no naturally occurring source of hydrogen in useful quantities.[128]
Hydrogen can be burned to produce heat or combined with oxygen in fuel cells to generate electricity directly, with water being the only emissions at the point of usage. The overall lifecycle emissions of hydrogen depend on how it is produced. Nearly all of the world’s current supply of hydrogen is created from fossil fuels.[129][130] The main method is steam methane reforming, in which hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide.[131] While carbon capture and storage can remove a large fraction of these emissions, the overall carbon footprint of hydrogen from natural gas is difficult to assess as of 2021, in part because of emissions created in the production of the natural gas itself.[132]
Electricity can be used to split water molecules, producing sustainable hydrogen provided the electricity was generated sustainably. However, this electrolysis process is currently more expensive than creating hydrogen from methane and the efficiency of energy conversion is inherently low.[133] Hydrogen can be produced when there is a surplus of variable renewable electricity, then stored and used to generate heat or to re-generate electricity.[134] It can be further transformed into synthetic fuels such as ammonia and methanol.[135]
Innovation in hydrogen electrolysers could make large-scale production of hydrogen from electricity more cost-competitive.[136] There is potential for hydrogen to play a significant role in decarbonising energy systems because in certain sectors, replacing fossil fuels with direct use of electricity would be very difficult.[133] Hydrogen fuel can produce the intense heat required for industrial production of steel, cement, glass, and chemicals. For steelmaking, hydrogen can function as a clean energy carrier and simultaneously as a low-carbon catalyst replacing coal-derived coke.[137] Hydrogen used in transportation would burn relatively cleanly, with some NOx emissions,[138] but without carbon emissions.[139] Disadvantages of hydrogen as an energy carrier include high costs of storage and distribution due to hydrogen’s explosivity, its large volume compared to other fuels, and its tendency to make pipes brittle.[132] The infrastructure costs associated with full conversion to a hydrogen economy would be substantial.[140]
Semiconductor industry
Hydrogen is employed to saturate broken («dangling») bonds of amorphous silicon and amorphous carbon that helps stabilizing material properties.[141] It is also a potential electron donor in various oxide materials, including ZnO,[142][143] SnO2, CdO, MgO,[144] ZrO2, HfO2, La2O3, Y2O3, TiO2, SrTiO3, LaAlO3, SiO2, Al2O3, ZrSiO4, HfSiO4, and SrZrO3.[145]
Aerospace
Liquid hydrogen and liquid oxygen together serve as cryogenic fuel in liquid-propellant rockets, as in the Space Shuttle main engines.
Niche and evolving uses
- Shielding gas: Hydrogen is used as a shielding gas in welding methods such as atomic hydrogen welding.[146][147]
- Cryogenic research: Liquid H2 is used in cryogenic research, including superconductivity studies.[148]
- Buoyant lifting: Because H2 is lighter than air, having only 7% of the density of air, it was once widely used as a lifting gas in balloons and airships.[149]
- Leak detection: Pure or mixed with nitrogen (sometimes called forming gas), hydrogen is a tracer gas for detection of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries.[150] Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.[151]
- Neutron moderation: Deuterium (hydrogen-2) is used in nuclear fission applications as a moderator to slow neutrons.
- Nuclear fusion fuel: Deuterium is used in nuclear fusion reactions.[6]
- Isotopic labeling: Deuterium compounds have applications in chemistry and biology in studies of isotope effects on reaction rates.[152]
- Rocket propellant: NASA has investigated the use of rocket propellant made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles that are suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.[153]
- Tritium uses: Tritium (hydrogen-3), produced in nuclear reactors, is used in the production of hydrogen bombs,[154] as an isotopic label in the biosciences,[64] and as a source of beta radiation in radioluminescent paint for instrument dials and emergency signage.[59]
Biological reactions
H2 is a product of some types of anaerobic metabolism and is produced by several microorganisms, usually via reactions catalyzed by iron- or nickel-containing enzymes called hydrogenases. These enzymes catalyze the reversible redox reaction between H2 and its component two protons and two electrons. Creation of hydrogen gas occurs in the transfer of reducing equivalents produced during pyruvate fermentation to water.[155] The natural cycle of hydrogen production and consumption by organisms is called the hydrogen cycle.[156] Hydrogen is the most abundant element in the human body in terms of numbers of atoms of the element but, it is the 3rd most abundant element by mass, because hydrogen is so light. H2 occurs in the breath of humans due to the metabolic activity of hydrogenase-containing microorganisms in the large intestine. The concentration in fasted people at rest is typically less than 5 parts per million (ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic hydrogen breath tests.[157]
Hydrogen gas is produced by some bacteria and algae and is a natural component of flatus, as is methane, itself a hydrogen source of increasing importance.[158]
Water splitting, in which water is decomposed into its component protons, electrons, and oxygen, occurs in the light reactions in all photosynthetic organisms. Some such organisms, including the alga Chlamydomonas reinhardtii and cyanobacteria, have evolved a second step in the dark reactions in which protons and electrons are reduced to form H2 gas by specialized hydrogenases in the chloroplast.[159] Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize H2 gas even in the presence of oxygen.[160] Efforts have also been undertaken with genetically modified alga in a bioreactor.[161]
Safety and precautions
| Hazards | |
|---|---|
| GHS labelling: | |
| Pictograms |  |
| Signal word | Danger |
| Hazard statements | H220 |
| Precautionary statements | P202, P210, P271, P377, P381, P403[162] |
| NFPA 704 (fire diamond) |
0 4 0 |
Hydrogen poses a number of hazards to human safety, from potential detonations and fires when mixed with air to being an asphyxiant in its pure, oxygen-free form.[163] In addition, liquid hydrogen is a cryogen and presents dangers (such as frostbite) associated with very cold liquids.[164] Hydrogen dissolves in many metals and in addition to leaking out, may have adverse effects on them, such as hydrogen embrittlement,[165] leading to cracks and explosions.[166] Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns.[167]
Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Many physical and chemical properties of hydrogen depend on the parahydrogen/orthohydrogen ratio (it often takes days or weeks at a given temperature to reach the equilibrium ratio, for which the data is usually given). Hydrogen detonation parameters, such as critical detonation pressure and temperature, strongly depend on the container geometry.[163]
See also
- Hydrogen economy – Using hydrogen to decarbonize sectors which are hard to electrify
- Hydrogen production – Family of industrial methods for generating hydrogen
- Hydrogen safety – Procedures for safe production, handling and use of hydrogen
- Hydrogen technologies – Technologies that relating to the production & use of hydrogen
- Hydrogen transport
- Liquid hydrogen – Liquid state of the element hydrogen
- Methane pyrolysis (for hydrogen)
- Natural hydrogen – Natural hydrogen, often called white hydrogen, is molecular hydrogen occurring in natural deposits
- Pyrolysis – Thermal decomposition of materials at elevated temperatures in an inert atmosphere
Notes
- ^ However, most of the universe’s mass is not in the form of baryons or chemical elements. See dark matter and dark energy.
- ^ 286 kJ/mol: energy per mole of the combustible material (molecular hydrogen).
References
- ^ «Standard Atomic Weights: Hydrogen». CIAAW. 2009.
- ^ Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. p. 240. ISBN 978-0123526519.
- ^ Lide, D. R., ed. (2005). «Magnetic susceptibility of the elements and inorganic compounds». CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 978-0-8493-0486-6.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 978-0-8493-0464-4.
- ^ a b c «Hydrogen». Van Nostrand’s Encyclopedia of Chemistry. Wylie-Interscience. 2005. pp. 797–799. ISBN 978-0-471-61525-5.
- ^ a b c d e f g h i j k l Emsley, John (2001). Nature’s Building Blocks. Oxford: Oxford University Press. pp. 183–191. ISBN 978-0-19-850341-5.
- ^ Stwertka, Albert (1996). A Guide to the Elements. Oxford University Press. pp. 16–21. ISBN 978-0-19-508083-4.
- ^ «Hydrogen». Encyclopædia Britannica. Archived from the original on 24 December 2021. Retrieved 25 December 2021.
- ^ Boyd, Padi (19 July 2014). «What is the chemical composition of stars?». NASA. Archived from the original on 15 January 2015. Retrieved 5 February 2008.
- ^ Tanabashi et al. (2018) p. 358. Chpt. 21.4.1: «Big-Bang Cosmology» Archived 29 June 2021 at the Wayback Machine (Revised September 2017) by K.A. Olive and J.A. Peacock.[full citation needed]
- ^ Laursen, S.; Chang, J.; Medlin, W.; Gürmen, N.; Fogler, H. S. (27 July 2004). «An extremely brief introduction to computational quantum chemistry». Molecular Modeling in Chemical Engineering. University of Michigan. Archived from the original on 20 May 2015. Retrieved 4 May 2015.
- ^ Presenter: Professor Jim Al-Khalili (21 January 2010). «Discovering the Elements». Chemistry: A Volatile History. 25:40 minutes in. BBC. BBC Four. Archived from the original on 25 January 2010. Retrieved 9 February 2010.
- ^ a b Dincer, Ibrahim; Acar, Canan (14 September 2015). «Review and evaluation of hydrogen production methods for better sustainability». International Journal of Hydrogen Energy. 40 (34): 11094–11111. doi:10.1016/j.ijhydene.2014.12.035. ISSN 0360-3199. Archived from the original on 15 February 2022. Retrieved 4 February 2022.
- ^ «Hydrogen Basics – Production». Florida Solar Energy Center. 2007. Archived from the original on 18 February 2008. Retrieved 5 February 2008.
- ^ dos Santos, K. G.; Eckert, C. T.; De Rossi, E.; Bariccatti, R. A.; Frigo, E. P.; Lindino, C. A.; Alves, H. J. (2017). «Hydrogen production in the electrolysis of water in Brazil, a review». Renewable and Sustainable Energy Reviews. 68: 563–571. doi:10.1016/j.rser.2016.09.128.
- ^ a b Rogers, H. C. (1999). «Hydrogen Embrittlement of Metals». Science. 159 (3819): 1057–1064. Bibcode:1968Sci…159.1057R. doi:10.1126/science.159.3819.1057. PMID 17775040. S2CID 19429952.
- ^ «Dihydrogen». O=CHem Directory. University of Southern Maine. Archived from the original on 13 February 2009. Retrieved 6 April 2009.
- ^ Committee on Alternatives and Strategies for Future Hydrogen Production and Use (2004). The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs. National Academies Press. p. 240. ISBN 978-0-309-09163-3. Archived from the original on 29 January 2021. Retrieved 3 September 2020.
- ^ Carcassi, M. N.; Fineschi, F. (2005). «Deflagrations of H2–air and CH4–air lean mixtures in a vented multi-compartment environment». Energy. 30 (8): 1439–1451. doi:10.1016/j.energy.2004.02.012.
- ^ Patnaik, P. (2007). A Comprehensive Guide to the Hazardous Properties of Chemical Substances. Wiley-Interscience. p. 402. ISBN 978-0-471-71458-3. Archived from the original on 26 January 2021. Retrieved 3 September 2020.
- ^ Schefer, E. W.; Kulatilaka, W. D.; Patterson, B. D.; Settersten, T. B. (June 2009). «Visible emission of hydrogen flames». Combustion and Flame. 156 (6): 1234–1241. doi:10.1016/j.combustflame.2009.01.011. Archived from the original on 29 January 2021. Retrieved 30 June 2019.
- ^ «Myths about the Hindenburg Crash». Airships.net. Archived from the original on 20 April 2021. Retrieved 29 March 2021.
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ Clayton, D. D. (2003). Handbook of Isotopes in the Cosmos: Hydrogen to Gallium. Cambridge University Press. ISBN 978-0-521-82381-4.
- ^ NAAP Labs (2009). «Energy Levels». University of Nebraska Lincoln. Archived from the original on 11 May 2015. Retrieved 20 May 2015.
- ^ «photon wavelength 13.6 eV». Wolfram Alpha. 20 May 2015. Archived from the original on 12 May 2016. Retrieved 20 May 2015.
- ^ Stern, D. P. (16 May 2005). «The Atomic Nucleus and Bohr’s Early Model of the Atom». NASA Goddard Space Flight Center (mirror). Archived from the original on 17 October 2008. Retrieved 20 December 2007.
- ^ Stern, D. P. (13 February 2005). «Wave Mechanics». NASA Goddard Space Flight Center. Archived from the original on 13 May 2008. Retrieved 16 April 2008.
- ^ Staff (2003). «Hydrogen (H2) Properties, Uses, Applications: Hydrogen Gas and Liquid Hydrogen». Universal Industrial Gases, Inc. Archived from the original on 19 February 2008. Retrieved 5 February 2008.
- ^ Green, Richard A.; et al. (2012). «The theory and practice of hyperpolarization in magnetic resonance using parahydrogen». Prog. Nucl. Magn. Reson. Spectrosc. 67: 1–48. doi:10.1016/j.pnmrs.2012.03.001. PMID 23101588. Archived from the original on 28 August 2021. Retrieved 28 August 2021.
- ^ «Die Entdeckung des para-Wasserstoffs (The discovery of para-hydrogen)». Max-Planck-Institut für Biophysikalische Chemie (in German). Archived from the original on 16 November 2020. Retrieved 9 November 2020.
- ^ Milenko, Yu. Ya.; Sibileva, R. M.; Strzhemechny, M. A. (1997). «Natural ortho-para conversion rate in liquid and gaseous hydrogen». Journal of Low Temperature Physics. 107 (1–2): 77–92. Bibcode:1997JLTP..107…77M. doi:10.1007/BF02396837. S2CID 120832814.
- ^ Hritz, J. (March 2006). «CH. 6 – Hydrogen» (PDF). NASA Glenn Research Center Glenn Safety Manual, Document GRC-MQSA.001. NASA. Archived from the original (PDF) on 16 February 2008. Retrieved 5 February 2008.
- ^ Amos, Wade A. (1 November 1998). «Costs of Storing and Transporting Hydrogen» (PDF). National Renewable Energy Laboratory. pp. 6–9. Archived (PDF) from the original on 26 December 2014. Retrieved 19 May 2015.
- ^ Svadlenak, R. E.; Scott, A. B. (1957). «The Conversion of Ortho- to Parahydrogen on Iron Oxide-Zinc Oxide Catalysts». Journal of the American Chemical Society. 79 (20): 5385–5388. doi:10.1021/ja01577a013.
- ^ Clark, J. (2002). «The Acidity of the Hydrogen Halides». Chemguide. Archived from the original on 20 February 2008. Retrieved 9 March 2008.
- ^ Kimball, J. W. (7 August 2003). «Hydrogen». Kimball’s Biology Pages. Archived from the original on 4 March 2008. Retrieved 4 March 2008.
- ^ IUPAC Compendium of Chemical Terminology, Electronic version, Hydrogen Bond Archived 19 March 2008 at the Wayback Machine
- ^ Sandrock, G. (2 May 2002). «Metal-Hydrogen Systems». Sandia National Laboratories. Archived from the original on 24 February 2008. Retrieved 23 March 2008.
- ^ a b «Structure and Nomenclature of Hydrocarbons». Purdue University. Archived from the original on 11 June 2012. Retrieved 23 March 2008.
- ^ «Organic Chemistry». Dictionary.com. Lexico Publishing Group. 2008. Archived from the original on 18 April 2008. Retrieved 23 March 2008.
- ^ «Biochemistry». Dictionary.com. Lexico Publishing Group. 2008. Archived from the original on 29 March 2008. Retrieved 23 March 2008.
- ^ Takeshita, T.; Wallace, W. E.; Craig, R. S. (1974). «Hydrogen solubility in 1:5 compounds between yttrium or thorium and nickel or cobalt». Inorganic Chemistry. 13 (9): 2282–2283. doi:10.1021/ic50139a050.
- ^ Kirchheim, R.; Mutschele, T.; Kieninger, W.; Gleiter, H.; Birringer, R.; Koble, T. (1988). «Hydrogen in amorphous and nanocrystalline metals». Materials Science and Engineering. 99 (1–2): 457–462. doi:10.1016/0025-5416(88)90377-1.
- ^ Kirchheim, R. (1988). «Hydrogen solubility and diffusivity in defective and amorphous metals». Progress in Materials Science. 32 (4): 262–325. doi:10.1016/0079-6425(88)90010-2.
- ^ Christensen, C. H.; Nørskov, J. K.; Johannessen, T. (9 July 2005). «Making society independent of fossil fuels – Danish researchers reveal new technology». Technical University of Denmark. Archived from the original on 21 May 2015. Retrieved 19 May 2015.
- ^ Moers, K. (1920). «Investigations on the Salt Character of Lithium Hydride». Zeitschrift für Anorganische und Allgemeine Chemie. 113 (191): 179–228. doi:10.1002/zaac.19201130116. Archived (PDF) from the original on 24 August 2019. Retrieved 24 August 2019.
- ^ Downs, A. J.; Pulham, C. R. (1994). «The hydrides of aluminium, gallium, indium, and thallium: a re-evaluation». Chemical Society Reviews. 23 (3): 175–184. doi:10.1039/CS9942300175.
- ^ Hibbs, D. E.; Jones, C.; Smithies, N. A. (1999). «A remarkably stable indium trihydride complex: synthesis and characterisation of [InH3P(C6H11)3]». Chemical Communications (2): 185–186. doi:10.1039/a809279f.
- ^ a b c Miessler, G. L.; Tarr, D. A. (2003). Inorganic Chemistry (3rd ed.). Prentice Hall. ISBN 978-0-13-035471-6.
- ^ Okumura, A. M.; Yeh, L. I.; Myers, J. D.; Lee, Y. T. (1990). «Infrared spectra of the solvated hydronium ion: vibrational predissociation spectroscopy of mass-selected H3O+•(H2O)n•(H2)m«. Journal of Physical Chemistry. 94 (9): 3416–3427. doi:10.1021/j100372a014.
- ^ Perdoncin, G.; Scorrano, G. (1977). «Protonation Equilibria in Water at Several Temperatures of Alcohols, Ethers, Acetone, Dimethyl Sulfide, and Dimethyl Sulfoxide». Journal of the American Chemical Society. 99 (21): 6983–6986. doi:10.1021/ja00463a035.
- ^ Carrington, A.; McNab, I. R. (1989). «The infrared predissociation spectrum of triatomic hydrogen cation (H3+)». Accounts of Chemical Research. 22 (6): 218–222. doi:10.1021/ar00162a004.
- ^ Gurov, Y. B.; Aleshkin, D. V.; Behr, M. N.; Lapushkin, S. V.; Morokhov, P. V.; Pechkurov, V. A.; Poroshin, N. O.; Sandukovsky, V. G.; Tel’kushev, M. V.; Chernyshev, B. A.; Tschurenkova, T. D. (2004). «Spectroscopy of superheavy hydrogen isotopes in stopped-pion absorption by nuclei». Physics of Atomic Nuclei. 68 (3): 491–97. Bibcode:2005PAN….68..491G. doi:10.1134/1.1891200. S2CID 122902571.
- ^ Korsheninnikov, A.; Nikolskii, E.; Kuzmin, E.; Ozawa, A.; Morimoto, K.; Tokanai, F.; Kanungo, R.; Tanihata, I.; et al. (2003). «Experimental Evidence for the Existence of 7H and for a Specific Structure of 8He». Physical Review Letters. 90 (8): 082501. Bibcode:2003PhRvL..90h2501K. doi:10.1103/PhysRevLett.90.082501. PMID 12633420.
- ^ Urey, H. C.; Brickwedde, F. G.; Murphy, G. M. (1933). «Names for the Hydrogen Isotopes». Science. 78 (2035): 602–603. Bibcode:1933Sci….78..602U. doi:10.1126/science.78.2035.602. PMID 17797765.
- ^ Oda, Y.; Nakamura, H.; Yamazaki, T.; Nagayama, K.; Yoshida, M.; Kanaya, S.; Ikehara, M. (1992). «1H NMR studies of deuterated ribonuclease HI selectively labeled with protonated amino acids». Journal of Biomolecular NMR. 2 (2): 137–47. doi:10.1007/BF01875525. PMID 1330130. S2CID 28027551.
- ^ Broad, W. J. (11 November 1991). «Breakthrough in Nuclear Fusion Offers Hope for Power of Future». The New York Times. Archived from the original on 29 January 2021. Retrieved 12 February 2008.
- ^ a b Traub, R. J.; Jensen, J. A. (June 1995). «Tritium radioluminescent devices, Health and Safety Manual» (PDF). International Atomic Energy Agency. p. 2.4. Archived (PDF) from the original on 6 September 2015. Retrieved 20 May 2015.
- ^ Staff (15 November 2007). «Tritium». U.S. Environmental Protection Agency. Archived from the original on 2 January 2008. Retrieved 12 February 2008.
- ^ Nave, C. R. (2006). «Deuterium-Tritium Fusion». HyperPhysics. Georgia State University. Archived from the original on 16 March 2008. Retrieved 8 March 2008.
- ^ Kendall, C.; Caldwell, E. (1998). C. Kendall; J. J. McDonnell (eds.). «Chapter 2: Fundamentals of Isotope Geochemistry». Isotope Tracers in Catchment Hydrology. US Geological Survey: 51–86. doi:10.1016/B978-0-444-81546-0.50009-4. Archived from the original on 14 March 2008. Retrieved 8 March 2008.
- ^ «The Tritium Laboratory». University of Miami. 2008. Archived from the original on 28 February 2008. Retrieved 8 March 2008.
- ^ a b Holte, A. E.; Houck, M. A.; Collie, N. L. (2004). «Potential Role of Parasitism in the Evolution of Mutualism in Astigmatid Mites». Experimental and Applied Acarology. 25 (2): 97–107. doi:10.1023/A:1010655610575. PMID 11513367. S2CID 13159020.
- ^ van der Krogt, P. (5 May 2005). «Hydrogen». Elementymology & Elements Multidict. Archived from the original on 23 January 2010. Retrieved 20 December 2010.
- ^ § IR-3.3.2, Provisional Recommendations Archived 9 February 2016 at the Wayback Machine, Nomenclature of Inorganic Chemistry, Chemical Nomenclature and Structure Representation Division, IUPAC. Accessed on line 3 October 2007.
- ^ IUPAC (1997). «Muonium». In A.D. McNaught, A. Wilkinson (ed.). Compendium of Chemical Terminology (2nd ed.). Blackwell Scientific Publications. doi:10.1351/goldbook.M04069. ISBN 978-0-86542-684-9. Archived from the original on 13 March 2008. Retrieved 15 November 2016.
- ^ V.W. Hughes; et al. (1960). «Formation of Muonium and Observation of its Larmor Precession». Physical Review Letters. 5 (2): 63–65. Bibcode:1960PhRvL…5…63H. doi:10.1103/PhysRevLett.5.63.
- ^ Bondi, D.K.; Connor, J.N.L.; Manz, J.; Römelt, J. (20 October 1983). «Exact quantum and vibrationally adiabatic quantum, semiclassical and quasiclassical study of the collinear reactions Cl + MuCl, Cl + HCl, Cl + DCl». Molecular Physics. 50 (3): 467–488. Bibcode:1983MolPh..50..467B. doi:10.1080/00268978300102491. ISSN 0026-8976.
- ^ W.H. Koppenol; IUPAC (2001). «Names for muonium and hydrogen atoms and their ions» (PDF). Pure and Applied Chemistry. 73 (2): 377–380. doi:10.1351/pac200173020377. S2CID 97138983. Archived (PDF) from the original on 14 May 2011. Retrieved 15 November 2016.
- ^ Holman, Jack P. (2002). Heat transfer (9th ed.). New York, NY: McGraw-Hill. pp. 600–606. ISBN 0-07-240655-0. OCLC 46959719.
{{cite book}}: CS1 maint: date and year (link) - ^ Incropera 1 Dewitt 2 Bergman 3 Lavigne 4, Frank P. 1 David P. 2 Theodore L. 3 Adrienne S. 4 (2007). Fundamentals of heat and mass transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 978-0-471-45728-2. OCLC 62532755.
{{cite book}}: CS1 maint: date and year (link) - ^ Boyle, R. (1672). Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air, and about explosions, an hydrostatical discourse occasion’d by some objections of Dr. Henry More against some explications of new experiments made by the author of these tracts: To which is annex’t, an hydrostatical letter, dilucidating an experiment about a way of weighing water in water, new experiments, of the positive or relative levity of bodies under water, of the air’s spring on bodies under water, about the differing pressure of heavy solids and fluids. Printed for Richard Davis. pp. 64–65.
- ^ Winter, M. (2007). «Hydrogen: historical information». WebElements Ltd. Archived from the original on 10 April 2008. Retrieved 5 February 2008.
- ^ Musgrave, A. (1976). «Why did oxygen supplant phlogiston? Research programmes in the Chemical Revolution». In Howson, C. (ed.). Method and appraisal in the physical sciences. The Critical Background to Modern Science, 1800–1905. Cambridge University Press. doi:10.1017/CBO9780511760013. ISBN 978-0-521-21110-9. Retrieved 22 October 2011.
- ^ Cavendish, Henry (12 May 1766). «Three Papers, Containing Experiments on Factitious Air, by the Hon. Henry Cavendish, F. R. S.» Philosophical Transactions. 56: 141–184. Bibcode:1766RSPT…56..141C. doi:10.1098/rstl.1766.0019. JSTOR 105491.
- ^ Stwertka, Albert (1996). A Guide to the Elements. Oxford University Press. pp. 16–21. ISBN 978-0-19-508083-4.
- ^ National Electrical Manufacturers Association (1946). A chronological history of electrical development from 600 B.C. New York, N.Y., National Electrical Manufacturers Association. p. 102. Archived from the original on 4 March 2016. Retrieved 9 February 2016.
- ^ Stockel, J.F; j.d. Dunlop; Betz, F (1980). «NTS-2 Nickel-Hydrogen Battery Performance 31». Journal of Spacecraft and Rockets. 17: 31–34. Bibcode:1980JSpRo..17…31S. doi:10.2514/3.57704.
- ^ Jannette, A. G.; Hojnicki, J. S.; McKissock, D. B.; Fincannon, J.; Kerslake, T. W.; Rodriguez, C. D. (July 2002). Validation of international space station electrical performance model via on-orbit telemetry (PDF). IECEC ’02. 2002 37th Intersociety Energy Conversion Engineering Conference, 2002. pp. 45–50. doi:10.1109/IECEC.2002.1391972. hdl:2060/20020070612. ISBN 0-7803-7296-4. Archived (PDF) from the original on 14 May 2010. Retrieved 11 November 2011.
- ^ Anderson, P. M.; Coyne, J. W. (2002). A lightweight high reliability single battery power system for interplanetary spacecraft. Aerospace Conference Proceedings. Vol. 5. pp. 5–2433. doi:10.1109/AERO.2002.1035418. ISBN 978-0-7803-7231-3. S2CID 108678345.
- ^ «Mars Global Surveyor». Astronautix.com. Archived from the original on 10 August 2009. Retrieved 6 April 2009.
- ^ Lori Tyahla, ed. (7 May 2009). «Hubble servicing mission 4 essentials». NASA. Archived from the original on 13 March 2015. Retrieved 19 May 2015.
- ^ Hendrix, Susan (25 November 2008). Lori Tyahla (ed.). «Extending Hubble’s mission life with new batteries». NASA. Archived from the original on 5 March 2016. Retrieved 19 May 2015.
- ^ Crepeau, R. (1 January 2006). Niels Bohr: The Atomic Model. Great Scientific Minds. ISBN 978-1-4298-0723-4.
- ^ Berman, R.; Cooke, A. H.; Hill, R. W. (1956). «Cryogenics». Annual Review of Physical Chemistry. 7: 1–20. Bibcode:1956ARPC….7….1B. doi:10.1146/annurev.pc.07.100156.000245.
- ^ Charlton, Mike; Van Der Werf, Dirk Peter (1 March 2015). «Advances in antihydrogen physics». Science Progress. 98 (1): 34–62. doi:10.3184/003685015X14234978376369. PMID 25942774. S2CID 23581065.
- ^ Kellerbauer, Alban (29 January 2015). «Why Antimatter Matters». European Review. 23 (1): 45–56. doi:10.1017/S1062798714000532. S2CID 58906869. Archived from the original on 29 January 2021. Retrieved 11 January 2020.
- ^ Gagnon, S. «Hydrogen». Jefferson Lab. Archived from the original on 10 April 2008. Retrieved 5 February 2008.
- ^ Haubold, H.; Mathai, A. M. (15 November 2007). «Solar Thermonuclear Energy Generation». Columbia University. Archived from the original on 11 December 2011. Retrieved 12 February 2008.
- ^ «Hydrogen». mysite.du.edu. Archived from the original on 18 April 2009. Retrieved 20 April 2008.
- ^ Storrie-Lombardi, L. J.; Wolfe, A. M. (2000). «Surveys for z > 3 Damped Lyman-alpha Absorption Systems: the Evolution of Neutral Gas». Astrophysical Journal. 543 (2): 552–576. arXiv:astro-ph/0006044. Bibcode:2000ApJ…543..552S. doi:10.1086/317138. S2CID 120150880.
- ^ Dresselhaus, M.; et al. (15 May 2003). «Basic Research Needs for the Hydrogen Economy» (PDF). APS March Meeting Abstracts. Argonne National Laboratory, U.S. Department of Energy, Office of Science Laboratory. 2004: m1.001. Bibcode:2004APS..MAR.m1001D. Archived from the original (PDF) on 13 February 2008. Retrieved 5 February 2008.
- ^ McCall Group; Oka Group (22 April 2005). «H3+ Resource Center». Universities of Illinois and Chicago. Archived from the original on 11 October 2007. Retrieved 5 February 2008.
- ^ Helm, H.; et al. (2003), «Coupling of Bound States to Continuum States in Neutral Triatomic Hydrogen», Dissociative Recombination of Molecular Ions with Electrons, Department of Molecular and Optical Physics, University of Freiburg, Germany, pp. 275–288, doi:10.1007/978-1-4615-0083-4_27, ISBN 978-1-4613-4915-0
- ^ Thomassen, Magnus. «Cost reduction and performance increase of PEM electrolysers» (PDF). fch.europa.eu. FCH JU. Archived (PDF) from the original on 17 April 2018. Retrieved 22 April 2018.
- ^ Kruse, B.; Grinna, S.; Buch, C. (2002). «Hydrogen Status og Muligheter» (PDF). Bellona. Archived from the original (PDF) on 16 February 2008. Retrieved 12 February 2008.
- ^ Von Wald, Gregory A. (2020). «Optimization-based technoeconomic analysis of molten-media methane pyrolysis for reducing industrial sector CO2 emissions». Sustainable Energy & Fuels. Royal Society of Chemistry. 4 (9): 4598–4613. doi:10.1039/D0SE00427H. S2CID 225676190. Archived from the original on 8 November 2020. Retrieved 31 October 2020.
- ^ Schneider, Stefan (2020). «State of the Art of Hydrogen Production via Pyrolysis of Natural Gas». ChemBioEng Reviews. Wiley Online Library. 7 (5): 150–158. doi:10.1002/cben.202000014.
- ^ Cartwright, Jon. «The reaction that would give us clean fossil fuels forever». New Scientist. Archived from the original on 26 October 2020. Retrieved 30 October 2020.
- ^ Karlsruhe Institute of Technology. «Hydrogen from methane without CO2 emissions». Phys.Org. Phys.Org. Archived from the original on 21 October 2020. Retrieved 30 October 2020.
- ^ Crolius, Stephen H. (27 January 2017). «Methane to Ammonia via Pyrolysis». Ammonia Energy Association. Ammonia Energy Association. Archived from the original on 31 December 2020. Retrieved 19 October 2020.
- ^ Fialka, John. «Energy Department Looks to Boost Hydrogen Fuel for Big Trucks». E&E News. Scientific American. Archived from the original on 6 November 2020. Retrieved 7 November 2020.
- ^ CCJ News (13 August 2020). «How fuel cell trucks produce electric power and how they’re fueled». CCJ News. Commercial Carrier Journal. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Toyota. «Hydrogen Fuel-Cell Class 8 Truck». Hydrogen-Powered Truck Will Offer Heavy-Duty Capability and Clean Emissions. Toyota. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Colias, Mike (26 October 2020). «Auto Makers Shift Their Hydrogen Focus to Big Rigs». Wall Street Journal. Archived from the original on 26 October 2020. Retrieved 26 October 2020.
- ^ GE Turbines. «Hydrogen fueled power turbines». Hydrogen fueled gas turbines. General Electric. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Solar Turbines. «Hydrogen fueled power turbines». Power From Hydrogen Gas For Carbon Reduction. Solar Turbines. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Upham, D. Chester (2017). «Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon». Science. American Association for Advancement of Science. 358 (6365): 917–921. Bibcode:2017Sci…358..917U. doi:10.1126/science.aao5023. PMID 29146810. S2CID 206663568.
- ^ Clarke, Palmer (2020). «Dry reforming of methane catalyzed by molten metal alloys». Nature Catalysis. 3: 83–89. doi:10.1038/s41929-019-0416-2. S2CID 210862772. Archived from the original on 29 January 2021. Retrieved 31 October 2020.
- ^ BASF. «BASF researchers working on fundamentally new, low-carbon production processes, Methane Pyrolysis». United States Sustainability. BASF. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Gusev, Alexander. «KITT/IASS – Producing CO2 Free Hydrogen From Natural Gas For Energy Usage». European Energy Innovation. Institute for Advanced Sustainability Studies. Archived from the original on 29 January 2021. Retrieved 30 October 2020.
- ^ Fernandez, Sonia. «Researchers develop potentially low-cost, low-emissions technology that can convert methane without forming CO2». Phys-Org. American Institute of Physics. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Freyermuth, George H. «1934 Patent: «The manufacture of hydrogen from methane hydrocarbons by the action of steam at elevated temperature»«. Patent Full-Text Databases. United States Patent and Trademark Office. Archived from the original on 1 October 2021. Retrieved 30 October 2020.
- ^ Press, Roman J.; Santhanam, K. S. V.; Miri, Massoud J.; Bailey, Alla V.; Takacs, Gerald A. (2008). Introduction to hydrogen Technology. John Wiley & Sons. p. 249. ISBN 978-0-471-77985-8.
- ^ a b c Oxtoby, D. W. (2002). Principles of Modern Chemistry (5th ed.). Thomson Brooks/Cole. ISBN 978-0-03-035373-4.
- ^ «Hydrogen Properties, Uses, Applications». Universal Industrial Gases, Inc. 2007. Archived from the original on 27 March 2008. Retrieved 11 March 2008.
- ^ Funderburg, E. (2008). «Why Are Nitrogen Prices So High?». The Samuel Roberts Noble Foundation. Archived from the original on 9 May 2001. Retrieved 11 March 2008.
- ^ Lees, A. (2007). «Chemicals from salt». BBC. Archived from the original on 26 October 2007. Retrieved 11 March 2008.
- ^ Parmuzina, A.V.; Kravchenko, O.V. (2008). «Activation of aluminium metal to evolve hydrogen from water». International Journal of Hydrogen Energy. 33 (12): 3073–3076. doi:10.1016/j.ijhydene.2008.02.025.
- ^ Weimer, Al (25 May 2005). «Development of solar-powered thermochemical production of hydrogen from water» (PDF). Solar Thermochemical Hydrogen Generation Project. Archived (PDF) from the original on 17 April 2007. Retrieved 21 December 2008.
- ^ Perret, R. «Development of Solar-Powered Thermochemical Production of Hydrogen from Water, DOE Hydrogen Program, 2007» (PDF). Archived from the original (PDF) on 27 May 2010. Retrieved 17 May 2008.
- ^ Russell, M. J.; Hall, A. J.; Martin, W. (2010). «Serpentinization as a source of energy at the origin of life». Geobiology. 8 (5): 355–371. doi:10.1111/j.1472-4669.2010.00249.x. PMID 20572872. S2CID 41118603.
- ^ Schrenk, M. O.; Brazelton, W. J.; Lang, S. Q. (2013). «Serpentinization, Carbon, and Deep Life» (PDF). Reviews in Mineralogy and Geochemistry. 75 (1): 575–606. Bibcode:2013RvMG…75..575S. doi:10.2138/rmg.2013.75.18. S2CID 8600635.
- ^ Smil, Vaclav (2004). Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (1st ed.). Cambridge, MA: MIT. ISBN 978-0-262-69313-4.
- ^ Chemistry Operations (15 December 2003). «Hydrogen». Los Alamos National Laboratory. Archived from the original on 4 March 2011. Retrieved 5 February 2008.
- ^ «DOE Seeks Applicants for Solicitation on the Employment Effects of a Transition to a Hydrogen Economy». Hydrogen Program (Press release). US Department of Energy. 22 March 2006. Archived from the original on 19 July 2011. Retrieved 16 March 2008.
- ^ McCarthy, J. (31 December 1995). «Hydrogen». Stanford University. Archived from the original on 14 March 2008. Retrieved 14 March 2008.
- ^ Reed, Stanley; Ewing, Jack (13 July 2021). «Hydrogen Is One Answer to Climate Change. Getting It Is the Hard Part». The New York Times. ISSN 0362-4331. Archived from the original on 14 July 2021. Retrieved 14 July 2021.
- ^ IRENA (2019). Hydrogen: A renewable energy perspective (PDF). p. 9. ISBN 978-92-9260-151-5. Archived (PDF) from the original on 29 September 2021. Retrieved 17 October 2021..
- ^ Bonheure, Mike; Vandewalle, Laurien A.; Marin, Guy B.; Van Geem, Kevin M. (March 2021). «Dream or Reality? Electrification of the Chemical Process Industries». CEP Magazine. American Institute of Chemical Engineers. Archived from the original on 17 July 2021. Retrieved 6 July 2021.
- ^ a b Griffiths, Steve; Sovacool, Benjamin K.; Kim, Jinsoo; Bazilian, Morgan; et al. (2021). «Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options». Energy Research & Social Science. 80: 39. doi:10.1016/j.erss.2021.102208. ISSN 2214-6296. Archived from the original on 16 October 2021. Retrieved 11 September 2021.
- ^ a b Evans, Simon; Gabbatiss, Josh (30 November 2020). «In-depth Q&A: Does the world need hydrogen to solve climate change?». Carbon Brief. Archived from the original on 1 December 2020. Retrieved 1 December 2020.
- ^ Palys, Matthew J.; Daoutidis, Prodromos (2020). «Using hydrogen and ammonia for renewable energy storage: A geographically comprehensive techno-economic study». Computers & Chemical Engineering. 136: 106785. doi:10.1016/j.compchemeng.2020.106785. ISSN 0098-1354.
- ^ IRENA (2021). World Energy Transitions Outlook: 1.5°C Pathway (PDF). pp. 12, 22. ISBN 978-92-9260-334-2. Archived (PDF) from the original on 11 June 2021.
- ^ IEA (2021). Net Zero by 2050: A Roadmap for the Global Energy Sector (PDF). pp. 15, 75–76. Archived (PDF) from the original on 23 May 2021.
- ^ Blank, Thomas; Molly, Patrick (January 2020). «Hydrogen’s Decarbonization Impact for Industry» (PDF). Rocky Mountain Institute. pp. 2, 7, 8. Archived (PDF) from the original on 22 September 2020.
- ^ Heffel, J. W. (2002). «NOx emission and performance data for a hydrogen fueled internal combustion engine at 1500 rpm using exhaust gas recirculation». International Journal of Hydrogen Energy. 28 (8): 901–908. doi:10.1016/S0360-3199(02)00157-X.
- ^ «Carbon Capture Strategy Could Lead to Emission-Free Cars» (Press release). Georgia Tech. 11 February 2008. Archived from the original on 28 September 2013. Retrieved 16 March 2008.
- ^ Romm, J. J. (2004). The Hype About Hydrogen: Fact And Fiction in the Race To Save The Climate (1st ed.). Island Press. ISBN 978-1-55963-703-9.
- ^ Le Comber, P. G.; Jones, D. I.; Spear, W. E. (1977). «Hall effect and impurity conduction in substitutionally doped amorphous silicon». Philosophical Magazine. 35 (5): 1173–1187. Bibcode:1977PMag…35.1173C. doi:10.1080/14786437708232943.
- ^ Van de Walle, C. G. (2000). «Hydrogen as a cause of doping in zinc oxide» (PDF). Physical Review Letters. 85 (5): 1012–1015. Bibcode:2000PhRvL..85.1012V. doi:10.1103/PhysRevLett.85.1012. hdl:11858/00-001M-0000-0026-D0E6-E. PMID 10991462. Archived (PDF) from the original on 15 August 2017. Retrieved 1 August 2018.
- ^ Janotti, A.; Van De Walle, C. G. (2007). «Hydrogen multicentre bonds». Nature Materials. 6 (1): 44–47. Bibcode:2007NatMa…6…44J. doi:10.1038/nmat1795. PMID 17143265.
- ^ Kilic, C.; Zunger, Alex (2002). «n-type doping of oxides by hydrogen». Applied Physics Letters. 81 (1): 73–75. Bibcode:2002ApPhL..81…73K. doi:10.1063/1.1482783. S2CID 96415065. Archived from the original on 29 January 2021. Retrieved 16 December 2019.
- ^ Peacock, P. W.; Robertson, J. (2003). «Behavior of hydrogen in high dielectric constant oxide gate insulators». Applied Physics Letters. 83 (10): 2025–2027. Bibcode:2003ApPhL..83.2025P. doi:10.1063/1.1609245.
- ^ Durgutlu, A. (2003). «Experimental investigation of the effect of hydrogen in argon as a shielding gas on TIG welding of austenitic stainless steel». Materials & Design. 25 (1): 19–23. doi:10.1016/j.matdes.2003.07.004.
- ^ «Atomic Hydrogen Welding». Specialty Welds. 2007. Archived from the original on 16 July 2011.
- ^ Hardy, W. N. (2003). «From H2 to cryogenic H masers to HiTc superconductors: An unlikely but rewarding path». Physica C: Superconductivity. 388–389: 1–6. Bibcode:2003PhyC..388….1H. doi:10.1016/S0921-4534(02)02591-1.
- ^ Almqvist, Ebbe (2003). History of industrial gases. New York, N.Y.: Kluwer Academic/Plenum Publishers. pp. 47–56. ISBN 978-0-306-47277-0. Retrieved 20 May 2015.
- ^ Block, M. (3 September 2004). Hydrogen as Tracer Gas for Leak Detection. 16th WCNDT 2004. Montreal, Canada: Sensistor Technologies. Archived from the original on 8 January 2009. Retrieved 25 March 2008.
- ^ «Report from the Commission on Dietary Food Additive Intake» (PDF). European Union. Archived (PDF) from the original on 16 February 2008. Retrieved 5 February 2008.
- ^ Reinsch, J.; Katz, A.; Wean, J.; Aprahamian, G.; MacFarland, J. T. (1980). «The deuterium isotope effect upon the reaction of fatty acyl-CoA dehydrogenase and butyryl-CoA». J. Biol. Chem. 255 (19): 9093–97. doi:10.1016/S0021-9258(19)70531-6. PMID 7410413.
- ^ «NASA/TM—2002-211915: Solid Hydrogen Experiments for Atomic Propellants» (PDF). Archived (PDF) from the original on 9 July 2021. Retrieved 2 July 2021.
- ^ Bergeron, K. D. (2004). «The Death of no-dual-use». Bulletin of the Atomic Scientists. 60 (1): 15–17. Bibcode:2004BuAtS..60a..15B. doi:10.2968/060001004. Archived from the original on 19 April 2008. Retrieved 13 April 2008.
- ^ Cammack, R.; Robson, R. L. (2001). Hydrogen as a Fuel: Learning from Nature. Taylor & Francis Ltd. pp. 202–203. ISBN 978-0-415-24242-4. Archived from the original on 29 January 2021. Retrieved 3 September 2020.
- ^ Rhee, T. S.; Brenninkmeijer, C. A. M.; Röckmann, T. (19 May 2006). «The overwhelming role of soils in the global atmospheric hydrogen cycle» (PDF). Atmospheric Chemistry and Physics. 6 (6): 1611–1625. Bibcode:2006ACP…..6.1611R. doi:10.5194/acp-6-1611-2006. Archived (PDF) from the original on 24 August 2019. Retrieved 24 August 2019.
- ^ Eisenmann, Alexander; Amann, Anton; Said, Michael; Datta, Bettina; Ledochowski, Maximilian (2008). «Implementation and interpretation of hydrogen breath tests» (PDF). Journal of Breath Research. 2 (4): 046002. Bibcode:2008JBR…..2d6002E. doi:10.1088/1752-7155/2/4/046002. PMID 21386189. S2CID 31706721. Archived from the original (PDF) on 29 January 2021. Retrieved 26 December 2020.
- ^ Berger, W. H. (15 November 2007). «The Future of Methane». University of California, San Diego. Archived from the original on 24 April 2008. Retrieved 12 February 2008.
- ^ Kruse, O.; Rupprecht, J.; Bader, K.; Thomas-Hall, S.; Schenk, P. M.; Finazzi, G.; Hankamer, B. (2005). «Improved photobiological H2 production in engineered green algal cells» (PDF). The Journal of Biological Chemistry. 280 (40): 34170–7. doi:10.1074/jbc.M503840200. PMID 16100118. S2CID 5373909. Archived (PDF) from the original on 29 January 2021. Retrieved 24 August 2019.
- ^ Smith, Hamilton O.; Xu, Qing (2005). «IV.E.6 Hydrogen from Water in a Novel Recombinant Oxygen-Tolerant Cyanobacteria System» (PDF). FY2005 Progress Report. United States Department of Energy. Archived (PDF) from the original on 29 December 2016. Retrieved 6 August 2016.
- ^ Williams, C. (24 February 2006). «Pond life: the future of energy». Science. The Register. Archived from the original on 9 May 2011. Retrieved 24 March 2008.
- ^ «MyChem: Chemical» (PDF). Archived from the original (PDF) on 1 October 2018. Retrieved 1 October 2018.
- ^ a b Brown, W. J.; et al. (1997). «Safety Standard for Hydrogen and Hydrogen Systems» (PDF). NASA. NSS 1740.16. Archived (PDF) from the original on 1 May 2017. Retrieved 12 July 2017.
- ^ «Liquid Hydrogen MSDS» (PDF). Praxair, Inc. September 2004. Archived from the original (PDF) on 27 May 2008. Retrieved 16 April 2008.
- ^ «‘Bugs’ and hydrogen embrittlement». Science News. 128 (3): 41. 20 July 1985. doi:10.2307/3970088. JSTOR 3970088.
- ^ Hayes, B. «Union Oil Amine Absorber Tower». TWI. Archived from the original on 20 November 2008. Retrieved 29 January 2010.
- ^ Walker, James L.; Waltrip, John S.; Zanker, Adam (1988). «Lactic acid to magnesium supply-demand relationships». In John J. McKetta; William Aaron Cunningham (eds.). Encyclopedia of Chemical Processing and Design. Vol. 28. New York: Dekker. p. 186. ISBN 978-0-8247-2478-8. Retrieved 20 May 2015.
Further reading
- Chart of the Nuclides (17th ed.). Knolls Atomic Power Laboratory. 2010. ISBN 978-0-9843653-0-2.
- Ferreira-Aparicio, P.; Benito, M. J.; Sanz, J. L. (2005). «New Trends in Reforming Technologies: from Hydrogen Industrial Plants to Multifuel Microreformers». Catalysis Reviews. 47 (4): 491–588. doi:10.1080/01614940500364958. S2CID 95966974.
- Newton, David E. (1994). The Chemical Elements. New York: Franklin Watts. ISBN 978-0-531-12501-4.
- Rigden, John S. (2002). Hydrogen: The Essential Element. Cambridge, Massachusetts: Harvard University Press. ISBN 978-0-531-12501-4.
- Romm, Joseph J. (2004). The Hype about Hydrogen, Fact and Fiction in the Race to Save the Climate. Island Press. ISBN 978-1-55963-703-9.
- Scerri, Eric (2007). The Periodic System, Its Story and Its Significance. New York: Oxford University Press. ISBN 978-0-19-530573-9.
- Hydrogen safety covers the safe production, handling and use
External links
![]()
These audio files were created from a revision of this article dated 28 October 2006, and do not reflect subsequent edits.
- Basic Hydrogen Calculations of Quantum Mechanics
- Hydrogen at The Periodic Table of Videos (University of Nottingham)
- High temperature hydrogen phase diagram
- Wavefunction of hydrogen
 Purple glow in its plasma state | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | colorless gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(H) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | 1s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | (H2) 13.99 K (−259.16 °C, −434.49 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | (H2) 20.271 K (−252.879 °C, −423.182 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at STP) | 0.08988 g/L | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 0.07 g/cm3 (solid: 0.0763 g/cm3)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at b.p.) | 0.07099 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 13.8033 K, 7.041 kPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 32.938 K, 1.2858 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | (H2) 0.117 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | (H2) 0.904 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | (H2) 28.836 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, +1 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 31±5 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 120 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of hydrogen | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 1310 m/s (gas, 27 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.1805 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −3.98×10−6 cm3/mol (298 K)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 12385-13-6 1333-74-0 (H2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Henry Cavendish[5][6] (1766) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Named by | Antoine Lavoisier[7] (1783) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of hydrogen
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H2. It is colorless, odorless, tasteless,[8] non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.[9][note 1] Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons.
In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 years later during the recombination epoch, when the plasma had cooled enough for electrons to remain bound to protons.[10]
Hydrogen is nonmetallic (except it becomes metallic at extremely high pressures) and readily forms a single covalent bond with most nonmetallic elements, forming compounds such as water and nearly all organic compounds. Hydrogen plays a particularly important role in acid–base reactions because these reactions usually involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) where it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The H+ cation is simply a proton (symbol p) but its behavior in aqueous solutions and in ionic compounds involves screening of its electric charge by nearby polar molecules or anions. Because hydrogen is the only neutral atom for which the Schrödinger equation can be solved analytically,[11] the study of its energetics and chemical bonding has played a key role in the development of quantum mechanics.
Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. In 1766–1781, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance,[12] and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means «water-former».
Industrial production is mainly from steam reforming of natural gas, oil reforming, or coal gasification.[13] A small percentage is also produced using more energy-intensive methods such as the electrolysis of water.[13][14][15] Most hydrogen is used near the site of its production, the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. It can be burned to produce heat or combined with oxygen in fuel cells to generate electricity directly, with water being the only emissions at the point of usage. Hydrogen atoms (but not gaseous molecules) are problematic in metallurgy because they can embrittle many metals.[16]
Properties
Combustion
Combustion of hydrogen with the oxygen in the air. When the bottom cap is removed, allowing air to enter at the bottom, the hydrogen in the container rises out of top and burns as it mixes with the air.

Hydrogen gas (dihydrogen or molecular hydrogen)[17] is highly flammable:
- 2 H2(g) + O2(g) → 2 H2O(l) (572 kJ/2 mol = 286 kJ/mol = 141.865 MJ/kg)[note 2]
The enthalpy of combustion is −286 kJ/mol.[18]
Hydrogen gas forms explosive mixtures with air in concentrations from 4–74%[19] and with chlorine at 5–95%. The explosive reactions may be triggered by spark, heat, or sunlight. The hydrogen autoignition temperature, the temperature of spontaneous ignition in air, is 500 °C (932 °F).[20]
Flame
Pure hydrogen-oxygen flames emit ultraviolet light and with high oxygen mix are nearly invisible to the naked eye, as illustrated by the faint plume of the Space Shuttle Main Engine, compared to the highly visible plume of a Space Shuttle Solid Rocket Booster, which uses an ammonium perchlorate composite. The detection of a burning hydrogen leak may require a flame detector; such leaks can be very dangerous. Hydrogen flames in other conditions are blue, resembling blue natural gas flames.[21] The destruction of the Hindenburg airship was a notorious example of hydrogen combustion and the cause is still debated. The visible flames in the photographs were the result of carbon compounds in the airship skin burning.[22]
Reactants
H2 is unreactive compared to diatomic elements such as halogens or oxygen. The thermodynamic basis of this low reactivity is the very strong H–H bond, with a bond dissociation energy of 435.7 kJ/mol.[23] The kinetic basis of the low reactivity is the nonpolar nature of H2 and its weak polarizability. It spontaneously reacts with chlorine and fluorine to form hydrogen chloride and hydrogen fluoride, respectively.[24] The reactivity of H2 is strongly affected by the presence of metal catalysts. Thus, while mixtures of H2 with O2 or air combust readily when heated to at least 500 °C by a spark or flame, they do not react at room temperature in the absence of a catalyst.
Electron energy levels

Depiction of a hydrogen atom with size of central proton shown, and the atomic diameter shown as about twice the Bohr model radius (image not to scale)
The ground state energy level of the electron in a hydrogen atom is −13.6 eV,[25] which is equivalent to an ultraviolet photon of roughly 91 nm wavelength.[26]
The energy levels of hydrogen can be calculated fairly accurately using the Bohr model of the atom, which conceptualizes the electron as «orbiting» the proton in analogy to the Earth’s orbit of the Sun. However, the atomic electron and proton are held together by electromagnetic force, while planets and celestial objects are held by gravity. Because of the discretization of angular momentum postulated in early quantum mechanics by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.[27]
A more accurate description of the hydrogen atom comes from a purely quantum mechanical treatment that uses the Schrödinger equation, Dirac equation or Feynman path integral formulation to calculate the probability density of the electron around the proton.[28] The most complicated treatments allow for the small effects of special relativity and vacuum polarization. In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum at all—illustrating how the «planetary orbit» differs from electron motion.
Spin isomers
Molecular H2 exists as two spin isomers, i.e. compounds that differ only in the spin states of their nuclei.[29] In the orthohydrogen form, the spins of the two nuclei are parallel, forming a spin triplet state having a total molecular spin 

The ortho-to-para ratio in H2 is an important consideration in the liquefaction and storage of liquid hydrogen: the conversion from ortho to para is exothermic and produces enough heat to evaporate a most of the liquid if not converted first to parahydrogen during the cooling process.[34] Catalysts for the ortho-para interconversion, such as ferric oxide and activated carbon compounds, are used during hydrogen cooling to avoid this loss of liquid.[35]
Phases

Hydrogen gas is colorless and transparent, here contained in a glass ampoule.

- Gaseous hydrogen
- Liquid hydrogen
- Slush hydrogen
- Solid hydrogen
- Metallic hydrogen
- Plasma hydrogen
Compounds
Covalent and organic compounds
While H2 is not very reactive under standard conditions, it does form compounds with most elements. Hydrogen can form compounds with elements that are more electronegative, such as halogens (F, Cl, Br, I), or oxygen; in these compounds hydrogen takes on a partial positive charge.[36] When bonded to a more electronegative element, particularly fluorine, oxygen, or nitrogen, hydrogen can participate in a form of medium-strength noncovalent bonding with another electronegative element with a lone pair, a phenomenon called hydrogen bonding that is critical to the stability of many biological molecules.[37][38] Hydrogen also forms compounds with less electronegative elements, such as metals and metalloids, where it takes on a partial negative charge. These compounds are often known as hydrides.[39]
Hydrogen forms a vast array of compounds with carbon called the hydrocarbons, and an even vaster array with heteroatoms that, because of their general association with living things, are called organic compounds.[40] The study of their properties is known as organic chemistry[41] and their study in the context of living organisms is known as biochemistry.[42] By some definitions, «organic» compounds are only required to contain carbon. However, most of them also contain hydrogen, and because it is the carbon-hydrogen bond that gives this class of compounds most of its particular chemical characteristics, carbon-hydrogen bonds are required in some definitions of the word «organic» in chemistry.[40] Millions of hydrocarbons are known, and they are usually formed by complicated pathways that seldom involve elemental hydrogen.
Hydrogen is highly soluble in many rare earth and transition metals[43] and is soluble in both nanocrystalline and amorphous metals.[44] Hydrogen solubility in metals is influenced by local distortions or impurities in the crystal lattice.[45] These properties may be useful when hydrogen is purified by passage through hot palladium disks, but the gas’s high solubility is a metallurgical problem, contributing to the embrittlement of many metals,[16] complicating the design of pipelines and storage tanks.[46]
Hydrides

Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. The term «hydride» suggests that the H atom has acquired a negative or anionic character, denoted H−, and is used when hydrogen forms a compound with a more electropositive element. The existence of the hydride anion, suggested by Gilbert N. Lewis in 1916 for group 1 and 2 salt-like hydrides, was demonstrated by Moers in 1920 by the electrolysis of molten lithium hydride (LiH), producing a stoichiometric quantity of hydrogen at the anode.[47] For hydrides other than group 1 and 2 metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group 2 hydrides is BeH2, which is polymeric. In lithium aluminium hydride, the [AlH4]− anion carries hydridic centers firmly attached to the Al(III).
Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, more than 100 binary borane hydrides are known, but only one binary aluminium hydride.[48] Binary indium hydride has not yet been identified, although larger complexes exist.[49]
In inorganic chemistry, hydrides can also serve as bridging ligands that link two metal centers in a coordination complex. This function is particularly common in group 13 elements, especially in boranes (boron hydrides) and aluminium complexes, as well as in clustered carboranes.[50]
Protons and acids
Oxidation of hydrogen removes its electron and gives H+, which contains no electrons and a nucleus which is usually composed of one proton. That is why H+ is often called a proton. This species is central to discussion of acids. Under the Brønsted–Lowry acid–base theory, acids are proton donors, while bases are proton acceptors.
A bare proton, H+, cannot exist in solution or in ionic crystals because of its unstoppable attraction to other atoms or molecules with electrons. Except at the high temperatures associated with plasmas, such protons cannot be removed from the electron clouds of atoms and molecules, and will remain attached to them. However, the term ‘proton’ is sometimes used loosely and metaphorically to refer to positively charged or cationic hydrogen attached to other species in this fashion, and as such is denoted «H+» without any implication that any single protons exist freely as a species.
To avoid the implication of the naked «solvated proton» in solution, acidic aqueous solutions are sometimes considered to contain a less unlikely fictitious species, termed the «hydronium ion» ([H3O]+). However, even in this case, such solvated hydrogen cations are more realistically conceived as being organized into clusters that form species closer to [H9O4]+.[51] Other oxonium ions are found when water is in acidic solution with other solvents.[52]
Although exotic on Earth, one of the most common ions in the universe is the H+3 ion, known as protonated molecular hydrogen or the trihydrogen cation.[53]
Isotopes


Hydrogen discharge (spectrum) tube

Deuterium discharge (spectrum) tube
Hydrogen has three naturally occurring isotopes, denoted 1
H, 2
H and 3
H. Other, highly unstable nuclei (4
H to 7
H) have been synthesized in the laboratory but not observed in nature.[54][55]
- 1
H is the most common hydrogen isotope, with an abundance of more than 99.98%. Because the nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name protium.[56] It is unique among all stable isotopes in having no neutrons; see diproton for a discussion of why others do not exist. - 2
H, the other stable hydrogen isotope, is known as deuterium and contains one proton and one neutron in the nucleus. All deuterium in the universe is thought to have been produced at the time of the Big Bang, and has endured since that time. Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called heavy water. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for 1
H-NMR spectroscopy.[57] Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion.[58] - 3
H is known as tritium and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into helium-3 through beta decay with a half-life of 12.32 years.[50] It is so radioactive that it can be used in luminous paint, making it useful in such things as watches. The glass prevents the small amount of radiation from getting out.[59] Small amounts of tritium are produced naturally by the interaction of cosmic rays with atmospheric gases; tritium has also been released during nuclear weapons tests.[60] It is used in nuclear fusion reactions,[61] as a tracer in isotope geochemistry,[62] and in specialized self-powered lighting devices.[63] Tritium has also been used in chemical and biological labeling experiments as a radiolabel.[64]
Unique among the elements, distinct names are assigned to its isotopes in common use today. During the early study of radioactivity, various heavy radioactive isotopes were given their own names, but such names are no longer used, except for deuterium and tritium. The symbols D and T (instead of 2
H and 3
H) are sometimes used for deuterium and tritium, but the symbol P is already in use for phosphorus and thus is not available for protium.[65] In its nomenclatural guidelines, the International Union of Pure and Applied Chemistry (IUPAC) allows any of D, T, 2
H, and 3
H to be used, although 2
H and 3
H are preferred.[66]
The exotic atom muonium (symbol Mu), composed of an antimuon and an electron, can also be considered a light radioisotope of hydrogen.[67] Because muons decay with lifetime 2.2 µs, muonium is too unstable to exhibit observable chemistry.[68] Nevertheless, muonium compounds are important test cases for quantum simulation, due to the mass difference between the antimuon and the proton,[69] and IUPAC nomenclature incorporates such hypothetical compounds as muonium chloride (MuCl) and sodium muonide (NaMu), analogous to hydrogen chloride and sodium hydride respectively.[70]
Thermal and physical properties
Table of thermal and physical properties of hydrogen (H2) at atmospheric pressure:[71][72]
| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg °C) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Thermal conductivity (W/m °C) | Thermal diffusivity (m^2/s) | Prandtl Number |
| 100 | 0.24255 | 11.23 | 4.21E-06 | 1.74E-05 | 6.70E-02 | 2.46E-05 | 0.707 |
| 150 | 0.16371 | 12.602 | 5.60E-06 | 3.42E-05 | 0.0981 | 4.75E-05 | 0.718 |
| 200 | 0.1227 | 13.54 | 6.81E-06 | 5.55E-05 | 0.1282 | 7.72E-05 | 0.719 |
| 250 | 0.09819 | 14.059 | 7.92E-06 | 8.06E-05 | 0.1561 | 1.13E-04 | 0.713 |
| 300 | 0.08185 | 14.314 | 8.96E-06 | 1.10E-04 | 0.182 | 1.55E-04 | 0.706 |
| 350 | 0.07016 | 14.436 | 9.95E-06 | 1.42E-04 | 0.206 | 2.03E-04 | 0.697 |
| 400 | 0.06135 | 14.491 | 1.09E-05 | 1.77E-04 | 0.228 | 2.57E-04 | 0.69 |
| 450 | 0.05462 | 14.499 | 1.18E-05 | 2.16E-04 | 0.251 | 3.16E-04 | 0.682 |
| 500 | 0.04918 | 14.507 | 1.26E-05 | 2.57E-04 | 0.272 | 3.82E-04 | 0.675 |
| 550 | 0.04469 | 14.532 | 1.35E-05 | 3.02E-04 | 0.292 | 4.52E-04 | 0.668 |
| 600 | 0.04085 | 14.537 | 1.43E-05 | 3.50E-04 | 0.315 | 5.31E-04 | 0.664 |
| 700 | 0.03492 | 14.574 | 1.59E-05 | 4.55E-04 | 0.351 | 6.90E-04 | 0.659 |
| 800 | 0.0306 | 14.675 | 1.74E-05 | 5.69E-04 | 0.384 | 8.56E-04 | 0.664 |
| 900 | 0.02723 | 14.821 | 1.88E-05 | 6.90E-04 | 0.412 | 1.02E-03 | 0.676 |
| 1000 | 0.02424 | 14.99 | 2.01E-05 | 8.30E-04 | 0.448 | 1.23E-03 | 0.673 |
| 1100 | 0.02204 | 15.17 | 2.13E-05 | 9.66E-04 | 0.488 | 1.46E-03 | 0.662 |
| 1200 | 0.0202 | 15.37 | 2.26E-05 | 1.12E-03 | 0.528 | 1.70E-03 | 0.659 |
| 1300 | 0.01865 | 15.59 | 2.39E-05 | 1.28E-03 | 0.568 | 1.96E-03 | 0.655 |
| 1400 | 0.01732 | 15.81 | 2.51E-05 | 1.45E-03 | 0.61 | 2.23E-03 | 0.65 |
| 1500 | 0.01616 | 16.02 | 2.63E-05 | 1.63E-03 | 0.655 | 2.53E-03 | 0.643 |
| 1600 | 0.0152 | 16.28 | 2.74E-05 | 1.80E-03 | 0.697 | 2.82E-03 | 0.639 |
| 1700 | 0.0143 | 16.58 | 2.85E-05 | 1.99E-03 | 0.742 | 3.13E-03 | 0.637 |
| 1800 | 0.0135 | 16.96 | 2.96E-05 | 2.19E-03 | 0.786 | 3.44E-03 | 0.639 |
| 1900 | 0.0128 | 17.49 | 3.07E-05 | 2.40E-03 | 0.835 | 3.73E-03 | 0.643 |
| 2000 | 0.0121 | 18.25 | 3.18E-05 | 2.63E-03 | 0.878 | 3.98E-03 | 0.661 |
History
Discovery and use
In 1671, Robert Boyle discovered and described the reaction between iron filings and dilute acids, which results in the production of hydrogen gas.[73][74]
Having provided a saline spirit [hydrochloric acid], which by an uncommon way of preparation was made exceeding sharp and piercing, we put into a vial, capable of containing three or four ounces of water, a convenient quantity of filings of steel, which were not such as are commonly sold in shops to Chymists and Apothecaries, (those being usually not free enough from rust) but such as I had a while before caus’d to be purposely fil’d off from a piece of good steel. This metalline powder being moistn’d in the viol with a little of the menstruum, was afterwards drench’d with more; whereupon the mixture grew very hot, and belch’d up copious and stinking fumes; which whether they consisted altogether of the volatile sulphur of the Mars [iron?], or of metalline steams participating of a sulphureous nature, and join’d with the saline exhalations of the menstruum, is not necessary to be here discuss’d. But whencesoever this stinking smoak proceeded, so inflammable it was, that upon the approach of a lighted candle to it, it would readily enough take fire, and burn with a blewish and somewhat greenish flame at the mouth of the viol for a good while together; and that, though with little light, yet with more strength than one would easily suspect.
— Robert Boyle, Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air…
In 1766, Henry Cavendish was the first to recognize hydrogen gas as a discrete substance, by naming the gas from a metal-acid reaction «inflammable air». He speculated that «inflammable air» was in fact identical to the hypothetical substance called «phlogiston»[75][76] and further finding in 1781 that the gas produces water when burned. He is usually given credit for the discovery of hydrogen as an element.[5][6] In 1783, Antoine Lavoisier gave the element the name hydrogen (from the Greek ὑδρο- hydro meaning «water» and -γενής genes meaning «former»)[77] when he and Laplace reproduced Cavendish’s finding that water is produced when hydrogen is burned.[6]

Antoine-Laurent de Lavoisier
Lavoisier produced hydrogen for his experiments on mass conservation by reacting a flux of steam with metallic iron through an incandescent iron tube heated in a fire. Anaerobic oxidation of iron by the protons of water at high temperature can be schematically represented by the set of following reactions:
- 1) Fe + H2O → FeO + H2
- 2) Fe + 3 H2O → Fe2O3 + 3 H2
- 3) Fe + 4 H2O → Fe3O4 + 4 H2
Many metals such as zirconium undergo a similar reaction with water leading to the production of hydrogen.
Hydrogen was liquefied for the first time by James Dewar in 1898 by using regenerative cooling and his invention, the vacuum flask.[6] He produced solid hydrogen the next year.[6] Deuterium was discovered in December 1931 by Harold Urey, and tritium was prepared in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck.[5] Heavy water, which consists of deuterium in the place of regular hydrogen, was discovered by Urey’s group in 1932.[6] François Isaac de Rivaz built the first de Rivaz engine, an internal combustion engine powered by a mixture of hydrogen and oxygen in 1806. Edward Daniel Clarke invented the hydrogen gas blowpipe in 1819. The Döbereiner’s lamp and limelight were invented in 1823.[6]
The first hydrogen-filled balloon was invented by Jacques Charles in 1783.[6] Hydrogen provided the lift for the first reliable form of air-travel following the 1852 invention of the first hydrogen-lifted airship by Henri Giffard.[6] German count Ferdinand von Zeppelin promoted the idea of rigid airships lifted by hydrogen that later were called Zeppelins; the first of which had its maiden flight in 1900.[6] Regularly scheduled flights started in 1910 and by the outbreak of World War I in August 1914, they had carried 35,000 passengers without a serious incident. Hydrogen-lifted airships were used as observation platforms and bombers during the war.
The first non-stop transatlantic crossing was made by the British airship R34 in 1919. Regular passenger service resumed in the 1920s and the discovery of helium reserves in the United States promised increased safety, but the U.S. government refused to sell the gas for this purpose. Therefore, H2 was used in the Hindenburg airship, which was destroyed in a midair fire over New Jersey on 6 May 1937.[6] The incident was broadcast live on radio and filmed. Ignition of leaking hydrogen is widely assumed to be the cause, but later investigations pointed to the ignition of the aluminized fabric coating by static electricity. But the damage to hydrogen’s reputation as a lifting gas was already done and commercial hydrogen airship travel ceased. Hydrogen is still used, in preference to non-flammable but more expensive helium, as a lifting gas for weather balloons.
In the same year, the first hydrogen-cooled turbogenerator went into service with gaseous hydrogen as a coolant in the rotor and the stator in 1937 at Dayton, Ohio, by the Dayton Power & Light Co.;[78] because of the thermal conductivity and very low viscosity of hydrogen gas, thus lower drag than air, this is the most common type in its field today for large generators (typically 60 MW and bigger; smaller generators are usually air-cooled).
The nickel hydrogen battery was used for the first time in 1977 aboard the U.S. Navy’s Navigation technology satellite-2 (NTS-2).[79] For example, the ISS,[80] Mars Odyssey[81] and the Mars Global Surveyor[82] are equipped with nickel-hydrogen batteries. In the dark part of its orbit, the Hubble Space Telescope is also powered by nickel-hydrogen batteries, which were finally replaced in May 2009,[83] more than 19 years after launch and 13 years beyond their design life.[84]
Role in quantum theory

Hydrogen emission spectrum lines in the visible range. These are the four visible lines of the Balmer series
Because of its simple atomic structure, consisting only of a proton and an electron, the hydrogen atom, together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of atomic structure.[85] Furthermore, study of the corresponding simplicity of the hydrogen molecule and the corresponding cation H+2 brought understanding of the nature of the chemical bond, which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s.
One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full quantum mechanical theory arrived. Maxwell observed that the specific heat capacity of H2 unaccountably departs from that of a diatomic gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in H2 because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.[86]
Antihydrogen (
H
) is the antimatter counterpart to hydrogen. It consists of an antiproton with a positron. Antihydrogen is the only type of antimatter atom to have been produced as of 2015.[87][88]
Cosmic prevalence and distribution

Hydrogen, as atomic H, is the most abundant chemical element in the universe, making up 75 percent of normal matter by mass and more than 90 percent by number of atoms. (Most of the mass of the universe, however, is not in the form of chemical-element type matter, but rather is postulated to occur as yet-undetected forms of mass such as dark matter and dark energy.[89]) This element is found in great abundance in stars and gas giant planets. Molecular clouds of H2 are associated with star formation. Hydrogen plays a vital role in powering stars through the proton-proton reaction in case of stars with very low to approximately 1 mass of the Sun and the CNO cycle of nuclear fusion in case of stars more massive than our Sun.[90]
States
Throughout the universe, hydrogen is mostly found in the atomic and plasma states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen’s electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind they interact with the Earth’s magnetosphere giving rise to Birkeland currents and the aurora.
Hydrogen is found in the neutral atomic state in the interstellar medium because the atoms seldom collide and combine. They are the source of the 21-cm hydrogen line at 1420 MHz that is detected in order to probe primordial hydrogen.[91] The large amount of neutral hydrogen found in the damped Lyman-alpha systems is thought to dominate the cosmological baryonic density of the universe up to a redshift of z = 4.[92]
Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, H2. Hydrogen gas is very rare in the Earth’s atmosphere (1 ppm by volume) because of its light weight, which enables it to escape from the atmosphere more rapidly than heavier gases. However, hydrogen is the third most abundant element on the Earth’s surface,[93] mostly in the form of chemical compounds such as hydrocarbons and water.[50]
A molecular form called protonated molecular hydrogen (H+3) is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic rays. This ion has also been observed in the upper atmosphere of the planet Jupiter. The ion is relatively stable in the environment of outer space due to the low temperature and density. H+3 is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium.[94] Neutral triatomic hydrogen H3 can exist only in an excited form and is unstable.[95] By contrast, the positive hydrogen molecular ion (H+2) is a rare molecule in the universe.
Production
H2 is produced in chemistry and biology laboratories, often as a by-product of other reactions; in industry for the hydrogenation of unsaturated substrates; and in nature as a means of expelling reducing equivalents in biochemical reactions.
Water electrolysis

Illustrating inputs and outputs of simple electrolysis of water production of hydrogen
The electrolysis of water is a simple method of producing hydrogen. A current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals. (Iron, for instance, would oxidize, and thus decrease the amount of oxygen given off.) The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is in the range 88–94%.[96][97]
- 2 H2O(l) → 2 H2(g) + O2(g)
Methane pyrolysis

Hydrogen production using natural gas methane pyrolysis is a one-step process that produces no greenhouse gases.[98][99][100][101] Developing volume production using this method is the key to enabling faster carbon reduction by using hydrogen in industrial processes,[102] fuel cell electric heavy truck transportation,[103][104][105][106] and in gas turbine electric power generation.[107][108] Methane pyrolysis is performed by having methane CH4 bubbled up through a molten metal catalyst containing dissolved nickel at 1,340 K (1,070 °C; 1,950 °F). This causes the methane to break down into hydrogen gas and solid carbon, with no other byproducts.[109][110]
- CH4(g) → C(s) + 2 H2(g) (ΔH° = 74 kJ/mol)
The industrial quality solid carbon may be sold as manufacturing feedstock or permanently landfilled; it is not released into the atmosphere and does not cause ground water pollution in landfill. Methane pyrolysis is in development and considered suitable for commercial bulk hydrogen production. Volume production is being evaluated in the BASF «methane pyrolysis at scale» pilot plant.[111] Further research continues in several laboratories, including at Karlsruhe Liquid-metal Laboratory (KALLA)[112] and the chemical engineering laboratory at University of California – Santa Barbara[113]
Other industrial methods

Illustrating inputs and outputs of steam reforming of natural gas, a process to produce hydrogen[image reference needed]
Hydrogen is often produced by reacting water with methane and carbon monoxide, which causes the removal of hydrogen from hydrocarbons at very high temperatures, with 48% of hydrogen production coming from steam reforming.[114][115] The water vapor is then reacted with the carbon monoxide produced by steam reforming to oxidize it to carbon dioxide and turn the water into hydrogen. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas[116] with release of atmospheric greenhouse gas or with capture using CCS and climate change mitigation. Steam reforming is also known as the Bosch process and is widely used for the industrial preparation of hydrogen.
At high temperatures (1000–1400 K, 700–1100 °C or 1300–2000 °F), steam (water vapor) reacts with methane to yield carbon monoxide and H2.
- CH4 + H2O → CO + 3 H2
This reaction is favored at low pressures but is nonetheless conducted at high pressures (2.0 MPa, 20 atm or 600 inHg). This is because high-pressure H2 is the most marketable product, and pressure swing adsorption (PSA) purification systems work better at higher pressures. The product mixture is known as «synthesis gas» because it is often used directly for the production of methanol and related compounds. Hydrocarbons other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
- CH4 → C + 2 H2
Consequently, steam reforming typically employs an excess of H2O. Additional hydrogen can be recovered from the steam by use of carbon monoxide through the water gas shift reaction, especially with an iron oxide catalyst. This reaction is also a common industrial source of carbon dioxide:[116]
- CO + H2O → CO2 + H2
Other important methods for CO and H2 production include partial oxidation of hydrocarbons:[117]
- 2 CH4 + O2 → 2 CO + 4 H2
and the coal reaction, which can serve as a prelude to the shift reaction above:[116]
- C + H2O → CO + H2
Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the Haber process for the production of ammonia, hydrogen is generated from natural gas.[118] Electrolysis of brine to yield chlorine also produces hydrogen as a co-product.[119]
Olefin production units may produce substantial quantities of byproduct hydrogen particularly from cracking light feedstocks like ethane or propane.
Metal-acid
Many metals react with water to produce H2, but the rate of hydrogen evolution depends on the metal, the pH, and the presence alloying agents. Most commonly, hydrogen evolution is induced by acids. The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids. This reaction is the basis of the Kipp’s apparatus, which once was used as a laboratory gas source:
- Zn + 2 H+ → Zn2+ + H2
In the absence of acid, the evolution of H2 is slower. Because iron is widely used structural material, its anaerobic corrosion is of technological significance:
- Fe + 2 H2O → Fe(OH)2 + H2
Many metals, such as aluminium, are slow to react with water because they form passivated coatings of oxides. An alloy of aluminium and gallium, however, does react with water.[120] At high pH, aluminium can produce H2:
- 2 Al + 6 H2O + 2 OH− → 2 [Al(OH)4]− + 3 H2
Some metal-containing compounds react with acids to evolve H2. Under anaerobic conditions, ferrous hydroxide (Fe(OH)
2) can be oxidized by the protons of water to form magnetite and H2. This process is described by the Schikorr reaction:
- 3 Fe(OH)2 → Fe3O4 + 2 H2O + H2
This process occurs during the anaerobic corrosion of iron and steel in oxygen-free groundwater and in reducing soils below the water table.
Thermochemical
More than 200 thermochemical cycles can be used for water splitting. Many of these cycles such as the iron oxide cycle, cerium(IV) oxide–cerium(III) oxide cycle, zinc zinc-oxide cycle, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle have been evaluated for their commercial potential to produce hydrogen and oxygen from water and heat without using electricity.[121] A number of laboratories (including in France, Germany, Greece, Japan, and the United States) are developing thermochemical methods to produce hydrogen from solar energy and water.[122]
Serpentinization reaction
In deep geological conditions prevailing far away from the Earth’s atmosphere, hydrogen (H2) is produced during the process of serpentinization. In this process, water protons (H+) are reduced by ferrous (Fe2+) ions provided by fayalite (Fe2SiO4). The reaction forms magnetite (Fe3O4), quartz (SiO2), and hydrogen (H2):[123][124]
- 3 Fe2SiO4 + 2 H2O → 2 Fe3O4 + 3 SiO2 + 3 H2
- fayalite + water → magnetite + quartz + hydrogen
This reaction closely resembles the Schikorr reaction observed in anaerobic oxidation of ferrous hydroxide in contact with water.
Applications
Petrochemical industry
Large quantities of H2 are used in the «upgrading» of fossil fuels. Key consumers of H2 include hydrodealkylation, hydrodesulfurization, and hydrocracking. Many of these reactions can be classified as hydrogenolysis, i.e., the cleavage of bonds to carbon. Illustrative is the separation of sulfur from liquid fossil fuels:
- R2S + 2 H2 → H2S + 2 RH
Hydrogenation
Hydrogenation, the addition of H2 to various substrates is conducted on a large scale. The hydrogenation of N2 to produce ammonia by the Haber–Bosch process consumes a few percent of the energy budget in the entire industry. The resulting ammonia is used to supply the majority of the protein consumed by humans.[125] Hydrogenation is used to convert unsaturated fats and oils to saturated fats and oils. The major application is the production of margarine. Methanol is produced by hydrogenation of carbon dioxide. It is similarly the source of hydrogen in the manufacture of hydrochloric acid. H2 is also used as a reducing agent for the conversion of some ores to the metals.[126]
Coolant
Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include low density, low viscosity, and the highest specific heat and thermal conductivity of all gases.
Energy carrier
Elemental hydrogen has been widely discussed in the context of energy, as a possible future carrier of energy on an economy-wide scale.[127] Hydrogen is a »carrier» of energy rather than an energy resource, because there is no naturally occurring source of hydrogen in useful quantities.[128]
Hydrogen can be burned to produce heat or combined with oxygen in fuel cells to generate electricity directly, with water being the only emissions at the point of usage. The overall lifecycle emissions of hydrogen depend on how it is produced. Nearly all of the world’s current supply of hydrogen is created from fossil fuels.[129][130] The main method is steam methane reforming, in which hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide.[131] While carbon capture and storage can remove a large fraction of these emissions, the overall carbon footprint of hydrogen from natural gas is difficult to assess as of 2021, in part because of emissions created in the production of the natural gas itself.[132]
Electricity can be used to split water molecules, producing sustainable hydrogen provided the electricity was generated sustainably. However, this electrolysis process is currently more expensive than creating hydrogen from methane and the efficiency of energy conversion is inherently low.[133] Hydrogen can be produced when there is a surplus of variable renewable electricity, then stored and used to generate heat or to re-generate electricity.[134] It can be further transformed into synthetic fuels such as ammonia and methanol.[135]
Innovation in hydrogen electrolysers could make large-scale production of hydrogen from electricity more cost-competitive.[136] There is potential for hydrogen to play a significant role in decarbonising energy systems because in certain sectors, replacing fossil fuels with direct use of electricity would be very difficult.[133] Hydrogen fuel can produce the intense heat required for industrial production of steel, cement, glass, and chemicals. For steelmaking, hydrogen can function as a clean energy carrier and simultaneously as a low-carbon catalyst replacing coal-derived coke.[137] Hydrogen used in transportation would burn relatively cleanly, with some NOx emissions,[138] but without carbon emissions.[139] Disadvantages of hydrogen as an energy carrier include high costs of storage and distribution due to hydrogen’s explosivity, its large volume compared to other fuels, and its tendency to make pipes brittle.[132] The infrastructure costs associated with full conversion to a hydrogen economy would be substantial.[140]
Semiconductor industry
Hydrogen is employed to saturate broken («dangling») bonds of amorphous silicon and amorphous carbon that helps stabilizing material properties.[141] It is also a potential electron donor in various oxide materials, including ZnO,[142][143] SnO2, CdO, MgO,[144] ZrO2, HfO2, La2O3, Y2O3, TiO2, SrTiO3, LaAlO3, SiO2, Al2O3, ZrSiO4, HfSiO4, and SrZrO3.[145]
Aerospace
Liquid hydrogen and liquid oxygen together serve as cryogenic fuel in liquid-propellant rockets, as in the Space Shuttle main engines.
Niche and evolving uses
- Shielding gas: Hydrogen is used as a shielding gas in welding methods such as atomic hydrogen welding.[146][147]
- Cryogenic research: Liquid H2 is used in cryogenic research, including superconductivity studies.[148]
- Buoyant lifting: Because H2 is lighter than air, having only 7% of the density of air, it was once widely used as a lifting gas in balloons and airships.[149]
- Leak detection: Pure or mixed with nitrogen (sometimes called forming gas), hydrogen is a tracer gas for detection of minute leaks. Applications can be found in the automotive, chemical, power generation, aerospace, and telecommunications industries.[150] Hydrogen is an authorized food additive (E 949) that allows food package leak testing, as well as having anti-oxidizing properties.[151]
- Neutron moderation: Deuterium (hydrogen-2) is used in nuclear fission applications as a moderator to slow neutrons.
- Nuclear fusion fuel: Deuterium is used in nuclear fusion reactions.[6]
- Isotopic labeling: Deuterium compounds have applications in chemistry and biology in studies of isotope effects on reaction rates.[152]
- Rocket propellant: NASA has investigated the use of rocket propellant made from atomic hydrogen, boron or carbon that is frozen into solid molecular hydrogen particles that are suspended in liquid helium. Upon warming, the mixture vaporizes to allow the atomic species to recombine, heating the mixture to high temperature.[153]
- Tritium uses: Tritium (hydrogen-3), produced in nuclear reactors, is used in the production of hydrogen bombs,[154] as an isotopic label in the biosciences,[64] and as a source of beta radiation in radioluminescent paint for instrument dials and emergency signage.[59]
Biological reactions
H2 is a product of some types of anaerobic metabolism and is produced by several microorganisms, usually via reactions catalyzed by iron- or nickel-containing enzymes called hydrogenases. These enzymes catalyze the reversible redox reaction between H2 and its component two protons and two electrons. Creation of hydrogen gas occurs in the transfer of reducing equivalents produced during pyruvate fermentation to water.[155] The natural cycle of hydrogen production and consumption by organisms is called the hydrogen cycle.[156] Hydrogen is the most abundant element in the human body in terms of numbers of atoms of the element but, it is the 3rd most abundant element by mass, because hydrogen is so light. H2 occurs in the breath of humans due to the metabolic activity of hydrogenase-containing microorganisms in the large intestine. The concentration in fasted people at rest is typically less than 5 parts per million (ppm) but can be 50 ppm when people with intestinal disorders consume molecules they cannot absorb during diagnostic hydrogen breath tests.[157]
Hydrogen gas is produced by some bacteria and algae and is a natural component of flatus, as is methane, itself a hydrogen source of increasing importance.[158]
Water splitting, in which water is decomposed into its component protons, electrons, and oxygen, occurs in the light reactions in all photosynthetic organisms. Some such organisms, including the alga Chlamydomonas reinhardtii and cyanobacteria, have evolved a second step in the dark reactions in which protons and electrons are reduced to form H2 gas by specialized hydrogenases in the chloroplast.[159] Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize H2 gas even in the presence of oxygen.[160] Efforts have also been undertaken with genetically modified alga in a bioreactor.[161]
Safety and precautions
| Hazards | |
|---|---|
| GHS labelling: | |
| Pictograms |  |
| Signal word | Danger |
| Hazard statements | H220 |
| Precautionary statements | P202, P210, P271, P377, P381, P403[162] |
| NFPA 704 (fire diamond) |
0 4 0 |
Hydrogen poses a number of hazards to human safety, from potential detonations and fires when mixed with air to being an asphyxiant in its pure, oxygen-free form.[163] In addition, liquid hydrogen is a cryogen and presents dangers (such as frostbite) associated with very cold liquids.[164] Hydrogen dissolves in many metals and in addition to leaking out, may have adverse effects on them, such as hydrogen embrittlement,[165] leading to cracks and explosions.[166] Hydrogen gas leaking into external air may spontaneously ignite. Moreover, hydrogen fire, while being extremely hot, is almost invisible, and thus can lead to accidental burns.[167]
Even interpreting the hydrogen data (including safety data) is confounded by a number of phenomena. Many physical and chemical properties of hydrogen depend on the parahydrogen/orthohydrogen ratio (it often takes days or weeks at a given temperature to reach the equilibrium ratio, for which the data is usually given). Hydrogen detonation parameters, such as critical detonation pressure and temperature, strongly depend on the container geometry.[163]
See also
- Hydrogen economy – Using hydrogen to decarbonize sectors which are hard to electrify
- Hydrogen production – Family of industrial methods for generating hydrogen
- Hydrogen safety – Procedures for safe production, handling and use of hydrogen
- Hydrogen technologies – Technologies that relating to the production & use of hydrogen
- Hydrogen transport
- Liquid hydrogen – Liquid state of the element hydrogen
- Methane pyrolysis (for hydrogen)
- Natural hydrogen – Natural hydrogen, often called white hydrogen, is molecular hydrogen occurring in natural deposits
- Pyrolysis – Thermal decomposition of materials at elevated temperatures in an inert atmosphere
Notes
- ^ However, most of the universe’s mass is not in the form of baryons or chemical elements. See dark matter and dark energy.
- ^ 286 kJ/mol: energy per mole of the combustible material (molecular hydrogen).
References
- ^ «Standard Atomic Weights: Hydrogen». CIAAW. 2009.
- ^ Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. p. 240. ISBN 978-0123526519.
- ^ Lide, D. R., ed. (2005). «Magnetic susceptibility of the elements and inorganic compounds». CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 978-0-8493-0486-6.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 978-0-8493-0464-4.
- ^ a b c «Hydrogen». Van Nostrand’s Encyclopedia of Chemistry. Wylie-Interscience. 2005. pp. 797–799. ISBN 978-0-471-61525-5.
- ^ a b c d e f g h i j k l Emsley, John (2001). Nature’s Building Blocks. Oxford: Oxford University Press. pp. 183–191. ISBN 978-0-19-850341-5.
- ^ Stwertka, Albert (1996). A Guide to the Elements. Oxford University Press. pp. 16–21. ISBN 978-0-19-508083-4.
- ^ «Hydrogen». Encyclopædia Britannica. Archived from the original on 24 December 2021. Retrieved 25 December 2021.
- ^ Boyd, Padi (19 July 2014). «What is the chemical composition of stars?». NASA. Archived from the original on 15 January 2015. Retrieved 5 February 2008.
- ^ Tanabashi et al. (2018) p. 358. Chpt. 21.4.1: «Big-Bang Cosmology» Archived 29 June 2021 at the Wayback Machine (Revised September 2017) by K.A. Olive and J.A. Peacock.[full citation needed]
- ^ Laursen, S.; Chang, J.; Medlin, W.; Gürmen, N.; Fogler, H. S. (27 July 2004). «An extremely brief introduction to computational quantum chemistry». Molecular Modeling in Chemical Engineering. University of Michigan. Archived from the original on 20 May 2015. Retrieved 4 May 2015.
- ^ Presenter: Professor Jim Al-Khalili (21 January 2010). «Discovering the Elements». Chemistry: A Volatile History. 25:40 minutes in. BBC. BBC Four. Archived from the original on 25 January 2010. Retrieved 9 February 2010.
- ^ a b Dincer, Ibrahim; Acar, Canan (14 September 2015). «Review and evaluation of hydrogen production methods for better sustainability». International Journal of Hydrogen Energy. 40 (34): 11094–11111. doi:10.1016/j.ijhydene.2014.12.035. ISSN 0360-3199. Archived from the original on 15 February 2022. Retrieved 4 February 2022.
- ^ «Hydrogen Basics – Production». Florida Solar Energy Center. 2007. Archived from the original on 18 February 2008. Retrieved 5 February 2008.
- ^ dos Santos, K. G.; Eckert, C. T.; De Rossi, E.; Bariccatti, R. A.; Frigo, E. P.; Lindino, C. A.; Alves, H. J. (2017). «Hydrogen production in the electrolysis of water in Brazil, a review». Renewable and Sustainable Energy Reviews. 68: 563–571. doi:10.1016/j.rser.2016.09.128.
- ^ a b Rogers, H. C. (1999). «Hydrogen Embrittlement of Metals». Science. 159 (3819): 1057–1064. Bibcode:1968Sci…159.1057R. doi:10.1126/science.159.3819.1057. PMID 17775040. S2CID 19429952.
- ^ «Dihydrogen». O=CHem Directory. University of Southern Maine. Archived from the original on 13 February 2009. Retrieved 6 April 2009.
- ^ Committee on Alternatives and Strategies for Future Hydrogen Production and Use (2004). The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs. National Academies Press. p. 240. ISBN 978-0-309-09163-3. Archived from the original on 29 January 2021. Retrieved 3 September 2020.
- ^ Carcassi, M. N.; Fineschi, F. (2005). «Deflagrations of H2–air and CH4–air lean mixtures in a vented multi-compartment environment». Energy. 30 (8): 1439–1451. doi:10.1016/j.energy.2004.02.012.
- ^ Patnaik, P. (2007). A Comprehensive Guide to the Hazardous Properties of Chemical Substances. Wiley-Interscience. p. 402. ISBN 978-0-471-71458-3. Archived from the original on 26 January 2021. Retrieved 3 September 2020.
- ^ Schefer, E. W.; Kulatilaka, W. D.; Patterson, B. D.; Settersten, T. B. (June 2009). «Visible emission of hydrogen flames». Combustion and Flame. 156 (6): 1234–1241. doi:10.1016/j.combustflame.2009.01.011. Archived from the original on 29 January 2021. Retrieved 30 June 2019.
- ^ «Myths about the Hindenburg Crash». Airships.net. Archived from the original on 20 April 2021. Retrieved 29 March 2021.
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ Clayton, D. D. (2003). Handbook of Isotopes in the Cosmos: Hydrogen to Gallium. Cambridge University Press. ISBN 978-0-521-82381-4.
- ^ NAAP Labs (2009). «Energy Levels». University of Nebraska Lincoln. Archived from the original on 11 May 2015. Retrieved 20 May 2015.
- ^ «photon wavelength 13.6 eV». Wolfram Alpha. 20 May 2015. Archived from the original on 12 May 2016. Retrieved 20 May 2015.
- ^ Stern, D. P. (16 May 2005). «The Atomic Nucleus and Bohr’s Early Model of the Atom». NASA Goddard Space Flight Center (mirror). Archived from the original on 17 October 2008. Retrieved 20 December 2007.
- ^ Stern, D. P. (13 February 2005). «Wave Mechanics». NASA Goddard Space Flight Center. Archived from the original on 13 May 2008. Retrieved 16 April 2008.
- ^ Staff (2003). «Hydrogen (H2) Properties, Uses, Applications: Hydrogen Gas and Liquid Hydrogen». Universal Industrial Gases, Inc. Archived from the original on 19 February 2008. Retrieved 5 February 2008.
- ^ Green, Richard A.; et al. (2012). «The theory and practice of hyperpolarization in magnetic resonance using parahydrogen». Prog. Nucl. Magn. Reson. Spectrosc. 67: 1–48. doi:10.1016/j.pnmrs.2012.03.001. PMID 23101588. Archived from the original on 28 August 2021. Retrieved 28 August 2021.
- ^ «Die Entdeckung des para-Wasserstoffs (The discovery of para-hydrogen)». Max-Planck-Institut für Biophysikalische Chemie (in German). Archived from the original on 16 November 2020. Retrieved 9 November 2020.
- ^ Milenko, Yu. Ya.; Sibileva, R. M.; Strzhemechny, M. A. (1997). «Natural ortho-para conversion rate in liquid and gaseous hydrogen». Journal of Low Temperature Physics. 107 (1–2): 77–92. Bibcode:1997JLTP..107…77M. doi:10.1007/BF02396837. S2CID 120832814.
- ^ Hritz, J. (March 2006). «CH. 6 – Hydrogen» (PDF). NASA Glenn Research Center Glenn Safety Manual, Document GRC-MQSA.001. NASA. Archived from the original (PDF) on 16 February 2008. Retrieved 5 February 2008.
- ^ Amos, Wade A. (1 November 1998). «Costs of Storing and Transporting Hydrogen» (PDF). National Renewable Energy Laboratory. pp. 6–9. Archived (PDF) from the original on 26 December 2014. Retrieved 19 May 2015.
- ^ Svadlenak, R. E.; Scott, A. B. (1957). «The Conversion of Ortho- to Parahydrogen on Iron Oxide-Zinc Oxide Catalysts». Journal of the American Chemical Society. 79 (20): 5385–5388. doi:10.1021/ja01577a013.
- ^ Clark, J. (2002). «The Acidity of the Hydrogen Halides». Chemguide. Archived from the original on 20 February 2008. Retrieved 9 March 2008.
- ^ Kimball, J. W. (7 August 2003). «Hydrogen». Kimball’s Biology Pages. Archived from the original on 4 March 2008. Retrieved 4 March 2008.
- ^ IUPAC Compendium of Chemical Terminology, Electronic version, Hydrogen Bond Archived 19 March 2008 at the Wayback Machine
- ^ Sandrock, G. (2 May 2002). «Metal-Hydrogen Systems». Sandia National Laboratories. Archived from the original on 24 February 2008. Retrieved 23 March 2008.
- ^ a b «Structure and Nomenclature of Hydrocarbons». Purdue University. Archived from the original on 11 June 2012. Retrieved 23 March 2008.
- ^ «Organic Chemistry». Dictionary.com. Lexico Publishing Group. 2008. Archived from the original on 18 April 2008. Retrieved 23 March 2008.
- ^ «Biochemistry». Dictionary.com. Lexico Publishing Group. 2008. Archived from the original on 29 March 2008. Retrieved 23 March 2008.
- ^ Takeshita, T.; Wallace, W. E.; Craig, R. S. (1974). «Hydrogen solubility in 1:5 compounds between yttrium or thorium and nickel or cobalt». Inorganic Chemistry. 13 (9): 2282–2283. doi:10.1021/ic50139a050.
- ^ Kirchheim, R.; Mutschele, T.; Kieninger, W.; Gleiter, H.; Birringer, R.; Koble, T. (1988). «Hydrogen in amorphous and nanocrystalline metals». Materials Science and Engineering. 99 (1–2): 457–462. doi:10.1016/0025-5416(88)90377-1.
- ^ Kirchheim, R. (1988). «Hydrogen solubility and diffusivity in defective and amorphous metals». Progress in Materials Science. 32 (4): 262–325. doi:10.1016/0079-6425(88)90010-2.
- ^ Christensen, C. H.; Nørskov, J. K.; Johannessen, T. (9 July 2005). «Making society independent of fossil fuels – Danish researchers reveal new technology». Technical University of Denmark. Archived from the original on 21 May 2015. Retrieved 19 May 2015.
- ^ Moers, K. (1920). «Investigations on the Salt Character of Lithium Hydride». Zeitschrift für Anorganische und Allgemeine Chemie. 113 (191): 179–228. doi:10.1002/zaac.19201130116. Archived (PDF) from the original on 24 August 2019. Retrieved 24 August 2019.
- ^ Downs, A. J.; Pulham, C. R. (1994). «The hydrides of aluminium, gallium, indium, and thallium: a re-evaluation». Chemical Society Reviews. 23 (3): 175–184. doi:10.1039/CS9942300175.
- ^ Hibbs, D. E.; Jones, C.; Smithies, N. A. (1999). «A remarkably stable indium trihydride complex: synthesis and characterisation of [InH3P(C6H11)3]». Chemical Communications (2): 185–186. doi:10.1039/a809279f.
- ^ a b c Miessler, G. L.; Tarr, D. A. (2003). Inorganic Chemistry (3rd ed.). Prentice Hall. ISBN 978-0-13-035471-6.
- ^ Okumura, A. M.; Yeh, L. I.; Myers, J. D.; Lee, Y. T. (1990). «Infrared spectra of the solvated hydronium ion: vibrational predissociation spectroscopy of mass-selected H3O+•(H2O)n•(H2)m«. Journal of Physical Chemistry. 94 (9): 3416–3427. doi:10.1021/j100372a014.
- ^ Perdoncin, G.; Scorrano, G. (1977). «Protonation Equilibria in Water at Several Temperatures of Alcohols, Ethers, Acetone, Dimethyl Sulfide, and Dimethyl Sulfoxide». Journal of the American Chemical Society. 99 (21): 6983–6986. doi:10.1021/ja00463a035.
- ^ Carrington, A.; McNab, I. R. (1989). «The infrared predissociation spectrum of triatomic hydrogen cation (H3+)». Accounts of Chemical Research. 22 (6): 218–222. doi:10.1021/ar00162a004.
- ^ Gurov, Y. B.; Aleshkin, D. V.; Behr, M. N.; Lapushkin, S. V.; Morokhov, P. V.; Pechkurov, V. A.; Poroshin, N. O.; Sandukovsky, V. G.; Tel’kushev, M. V.; Chernyshev, B. A.; Tschurenkova, T. D. (2004). «Spectroscopy of superheavy hydrogen isotopes in stopped-pion absorption by nuclei». Physics of Atomic Nuclei. 68 (3): 491–97. Bibcode:2005PAN….68..491G. doi:10.1134/1.1891200. S2CID 122902571.
- ^ Korsheninnikov, A.; Nikolskii, E.; Kuzmin, E.; Ozawa, A.; Morimoto, K.; Tokanai, F.; Kanungo, R.; Tanihata, I.; et al. (2003). «Experimental Evidence for the Existence of 7H and for a Specific Structure of 8He». Physical Review Letters. 90 (8): 082501. Bibcode:2003PhRvL..90h2501K. doi:10.1103/PhysRevLett.90.082501. PMID 12633420.
- ^ Urey, H. C.; Brickwedde, F. G.; Murphy, G. M. (1933). «Names for the Hydrogen Isotopes». Science. 78 (2035): 602–603. Bibcode:1933Sci….78..602U. doi:10.1126/science.78.2035.602. PMID 17797765.
- ^ Oda, Y.; Nakamura, H.; Yamazaki, T.; Nagayama, K.; Yoshida, M.; Kanaya, S.; Ikehara, M. (1992). «1H NMR studies of deuterated ribonuclease HI selectively labeled with protonated amino acids». Journal of Biomolecular NMR. 2 (2): 137–47. doi:10.1007/BF01875525. PMID 1330130. S2CID 28027551.
- ^ Broad, W. J. (11 November 1991). «Breakthrough in Nuclear Fusion Offers Hope for Power of Future». The New York Times. Archived from the original on 29 January 2021. Retrieved 12 February 2008.
- ^ a b Traub, R. J.; Jensen, J. A. (June 1995). «Tritium radioluminescent devices, Health and Safety Manual» (PDF). International Atomic Energy Agency. p. 2.4. Archived (PDF) from the original on 6 September 2015. Retrieved 20 May 2015.
- ^ Staff (15 November 2007). «Tritium». U.S. Environmental Protection Agency. Archived from the original on 2 January 2008. Retrieved 12 February 2008.
- ^ Nave, C. R. (2006). «Deuterium-Tritium Fusion». HyperPhysics. Georgia State University. Archived from the original on 16 March 2008. Retrieved 8 March 2008.
- ^ Kendall, C.; Caldwell, E. (1998). C. Kendall; J. J. McDonnell (eds.). «Chapter 2: Fundamentals of Isotope Geochemistry». Isotope Tracers in Catchment Hydrology. US Geological Survey: 51–86. doi:10.1016/B978-0-444-81546-0.50009-4. Archived from the original on 14 March 2008. Retrieved 8 March 2008.
- ^ «The Tritium Laboratory». University of Miami. 2008. Archived from the original on 28 February 2008. Retrieved 8 March 2008.
- ^ a b Holte, A. E.; Houck, M. A.; Collie, N. L. (2004). «Potential Role of Parasitism in the Evolution of Mutualism in Astigmatid Mites». Experimental and Applied Acarology. 25 (2): 97–107. doi:10.1023/A:1010655610575. PMID 11513367. S2CID 13159020.
- ^ van der Krogt, P. (5 May 2005). «Hydrogen». Elementymology & Elements Multidict. Archived from the original on 23 January 2010. Retrieved 20 December 2010.
- ^ § IR-3.3.2, Provisional Recommendations Archived 9 February 2016 at the Wayback Machine, Nomenclature of Inorganic Chemistry, Chemical Nomenclature and Structure Representation Division, IUPAC. Accessed on line 3 October 2007.
- ^ IUPAC (1997). «Muonium». In A.D. McNaught, A. Wilkinson (ed.). Compendium of Chemical Terminology (2nd ed.). Blackwell Scientific Publications. doi:10.1351/goldbook.M04069. ISBN 978-0-86542-684-9. Archived from the original on 13 March 2008. Retrieved 15 November 2016.
- ^ V.W. Hughes; et al. (1960). «Formation of Muonium and Observation of its Larmor Precession». Physical Review Letters. 5 (2): 63–65. Bibcode:1960PhRvL…5…63H. doi:10.1103/PhysRevLett.5.63.
- ^ Bondi, D.K.; Connor, J.N.L.; Manz, J.; Römelt, J. (20 October 1983). «Exact quantum and vibrationally adiabatic quantum, semiclassical and quasiclassical study of the collinear reactions Cl + MuCl, Cl + HCl, Cl + DCl». Molecular Physics. 50 (3): 467–488. Bibcode:1983MolPh..50..467B. doi:10.1080/00268978300102491. ISSN 0026-8976.
- ^ W.H. Koppenol; IUPAC (2001). «Names for muonium and hydrogen atoms and their ions» (PDF). Pure and Applied Chemistry. 73 (2): 377–380. doi:10.1351/pac200173020377. S2CID 97138983. Archived (PDF) from the original on 14 May 2011. Retrieved 15 November 2016.
- ^ Holman, Jack P. (2002). Heat transfer (9th ed.). New York, NY: McGraw-Hill. pp. 600–606. ISBN 0-07-240655-0. OCLC 46959719.
{{cite book}}: CS1 maint: date and year (link) - ^ Incropera 1 Dewitt 2 Bergman 3 Lavigne 4, Frank P. 1 David P. 2 Theodore L. 3 Adrienne S. 4 (2007). Fundamentals of heat and mass transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 978-0-471-45728-2. OCLC 62532755.
{{cite book}}: CS1 maint: date and year (link) - ^ Boyle, R. (1672). Tracts written by the Honourable Robert Boyle containing new experiments, touching the relation betwixt flame and air, and about explosions, an hydrostatical discourse occasion’d by some objections of Dr. Henry More against some explications of new experiments made by the author of these tracts: To which is annex’t, an hydrostatical letter, dilucidating an experiment about a way of weighing water in water, new experiments, of the positive or relative levity of bodies under water, of the air’s spring on bodies under water, about the differing pressure of heavy solids and fluids. Printed for Richard Davis. pp. 64–65.
- ^ Winter, M. (2007). «Hydrogen: historical information». WebElements Ltd. Archived from the original on 10 April 2008. Retrieved 5 February 2008.
- ^ Musgrave, A. (1976). «Why did oxygen supplant phlogiston? Research programmes in the Chemical Revolution». In Howson, C. (ed.). Method and appraisal in the physical sciences. The Critical Background to Modern Science, 1800–1905. Cambridge University Press. doi:10.1017/CBO9780511760013. ISBN 978-0-521-21110-9. Retrieved 22 October 2011.
- ^ Cavendish, Henry (12 May 1766). «Three Papers, Containing Experiments on Factitious Air, by the Hon. Henry Cavendish, F. R. S.» Philosophical Transactions. 56: 141–184. Bibcode:1766RSPT…56..141C. doi:10.1098/rstl.1766.0019. JSTOR 105491.
- ^ Stwertka, Albert (1996). A Guide to the Elements. Oxford University Press. pp. 16–21. ISBN 978-0-19-508083-4.
- ^ National Electrical Manufacturers Association (1946). A chronological history of electrical development from 600 B.C. New York, N.Y., National Electrical Manufacturers Association. p. 102. Archived from the original on 4 March 2016. Retrieved 9 February 2016.
- ^ Stockel, J.F; j.d. Dunlop; Betz, F (1980). «NTS-2 Nickel-Hydrogen Battery Performance 31». Journal of Spacecraft and Rockets. 17: 31–34. Bibcode:1980JSpRo..17…31S. doi:10.2514/3.57704.
- ^ Jannette, A. G.; Hojnicki, J. S.; McKissock, D. B.; Fincannon, J.; Kerslake, T. W.; Rodriguez, C. D. (July 2002). Validation of international space station electrical performance model via on-orbit telemetry (PDF). IECEC ’02. 2002 37th Intersociety Energy Conversion Engineering Conference, 2002. pp. 45–50. doi:10.1109/IECEC.2002.1391972. hdl:2060/20020070612. ISBN 0-7803-7296-4. Archived (PDF) from the original on 14 May 2010. Retrieved 11 November 2011.
- ^ Anderson, P. M.; Coyne, J. W. (2002). A lightweight high reliability single battery power system for interplanetary spacecraft. Aerospace Conference Proceedings. Vol. 5. pp. 5–2433. doi:10.1109/AERO.2002.1035418. ISBN 978-0-7803-7231-3. S2CID 108678345.
- ^ «Mars Global Surveyor». Astronautix.com. Archived from the original on 10 August 2009. Retrieved 6 April 2009.
- ^ Lori Tyahla, ed. (7 May 2009). «Hubble servicing mission 4 essentials». NASA. Archived from the original on 13 March 2015. Retrieved 19 May 2015.
- ^ Hendrix, Susan (25 November 2008). Lori Tyahla (ed.). «Extending Hubble’s mission life with new batteries». NASA. Archived from the original on 5 March 2016. Retrieved 19 May 2015.
- ^ Crepeau, R. (1 January 2006). Niels Bohr: The Atomic Model. Great Scientific Minds. ISBN 978-1-4298-0723-4.
- ^ Berman, R.; Cooke, A. H.; Hill, R. W. (1956). «Cryogenics». Annual Review of Physical Chemistry. 7: 1–20. Bibcode:1956ARPC….7….1B. doi:10.1146/annurev.pc.07.100156.000245.
- ^ Charlton, Mike; Van Der Werf, Dirk Peter (1 March 2015). «Advances in antihydrogen physics». Science Progress. 98 (1): 34–62. doi:10.3184/003685015X14234978376369. PMID 25942774. S2CID 23581065.
- ^ Kellerbauer, Alban (29 January 2015). «Why Antimatter Matters». European Review. 23 (1): 45–56. doi:10.1017/S1062798714000532. S2CID 58906869. Archived from the original on 29 January 2021. Retrieved 11 January 2020.
- ^ Gagnon, S. «Hydrogen». Jefferson Lab. Archived from the original on 10 April 2008. Retrieved 5 February 2008.
- ^ Haubold, H.; Mathai, A. M. (15 November 2007). «Solar Thermonuclear Energy Generation». Columbia University. Archived from the original on 11 December 2011. Retrieved 12 February 2008.
- ^ «Hydrogen». mysite.du.edu. Archived from the original on 18 April 2009. Retrieved 20 April 2008.
- ^ Storrie-Lombardi, L. J.; Wolfe, A. M. (2000). «Surveys for z > 3 Damped Lyman-alpha Absorption Systems: the Evolution of Neutral Gas». Astrophysical Journal. 543 (2): 552–576. arXiv:astro-ph/0006044. Bibcode:2000ApJ…543..552S. doi:10.1086/317138. S2CID 120150880.
- ^ Dresselhaus, M.; et al. (15 May 2003). «Basic Research Needs for the Hydrogen Economy» (PDF). APS March Meeting Abstracts. Argonne National Laboratory, U.S. Department of Energy, Office of Science Laboratory. 2004: m1.001. Bibcode:2004APS..MAR.m1001D. Archived from the original (PDF) on 13 February 2008. Retrieved 5 February 2008.
- ^ McCall Group; Oka Group (22 April 2005). «H3+ Resource Center». Universities of Illinois and Chicago. Archived from the original on 11 October 2007. Retrieved 5 February 2008.
- ^ Helm, H.; et al. (2003), «Coupling of Bound States to Continuum States in Neutral Triatomic Hydrogen», Dissociative Recombination of Molecular Ions with Electrons, Department of Molecular and Optical Physics, University of Freiburg, Germany, pp. 275–288, doi:10.1007/978-1-4615-0083-4_27, ISBN 978-1-4613-4915-0
- ^ Thomassen, Magnus. «Cost reduction and performance increase of PEM electrolysers» (PDF). fch.europa.eu. FCH JU. Archived (PDF) from the original on 17 April 2018. Retrieved 22 April 2018.
- ^ Kruse, B.; Grinna, S.; Buch, C. (2002). «Hydrogen Status og Muligheter» (PDF). Bellona. Archived from the original (PDF) on 16 February 2008. Retrieved 12 February 2008.
- ^ Von Wald, Gregory A. (2020). «Optimization-based technoeconomic analysis of molten-media methane pyrolysis for reducing industrial sector CO2 emissions». Sustainable Energy & Fuels. Royal Society of Chemistry. 4 (9): 4598–4613. doi:10.1039/D0SE00427H. S2CID 225676190. Archived from the original on 8 November 2020. Retrieved 31 October 2020.
- ^ Schneider, Stefan (2020). «State of the Art of Hydrogen Production via Pyrolysis of Natural Gas». ChemBioEng Reviews. Wiley Online Library. 7 (5): 150–158. doi:10.1002/cben.202000014.
- ^ Cartwright, Jon. «The reaction that would give us clean fossil fuels forever». New Scientist. Archived from the original on 26 October 2020. Retrieved 30 October 2020.
- ^ Karlsruhe Institute of Technology. «Hydrogen from methane without CO2 emissions». Phys.Org. Phys.Org. Archived from the original on 21 October 2020. Retrieved 30 October 2020.
- ^ Crolius, Stephen H. (27 January 2017). «Methane to Ammonia via Pyrolysis». Ammonia Energy Association. Ammonia Energy Association. Archived from the original on 31 December 2020. Retrieved 19 October 2020.
- ^ Fialka, John. «Energy Department Looks to Boost Hydrogen Fuel for Big Trucks». E&E News. Scientific American. Archived from the original on 6 November 2020. Retrieved 7 November 2020.
- ^ CCJ News (13 August 2020). «How fuel cell trucks produce electric power and how they’re fueled». CCJ News. Commercial Carrier Journal. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Toyota. «Hydrogen Fuel-Cell Class 8 Truck». Hydrogen-Powered Truck Will Offer Heavy-Duty Capability and Clean Emissions. Toyota. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Colias, Mike (26 October 2020). «Auto Makers Shift Their Hydrogen Focus to Big Rigs». Wall Street Journal. Archived from the original on 26 October 2020. Retrieved 26 October 2020.
- ^ GE Turbines. «Hydrogen fueled power turbines». Hydrogen fueled gas turbines. General Electric. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Solar Turbines. «Hydrogen fueled power turbines». Power From Hydrogen Gas For Carbon Reduction. Solar Turbines. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Upham, D. Chester (2017). «Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon». Science. American Association for Advancement of Science. 358 (6365): 917–921. Bibcode:2017Sci…358..917U. doi:10.1126/science.aao5023. PMID 29146810. S2CID 206663568.
- ^ Clarke, Palmer (2020). «Dry reforming of methane catalyzed by molten metal alloys». Nature Catalysis. 3: 83–89. doi:10.1038/s41929-019-0416-2. S2CID 210862772. Archived from the original on 29 January 2021. Retrieved 31 October 2020.
- ^ BASF. «BASF researchers working on fundamentally new, low-carbon production processes, Methane Pyrolysis». United States Sustainability. BASF. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Gusev, Alexander. «KITT/IASS – Producing CO2 Free Hydrogen From Natural Gas For Energy Usage». European Energy Innovation. Institute for Advanced Sustainability Studies. Archived from the original on 29 January 2021. Retrieved 30 October 2020.
- ^ Fernandez, Sonia. «Researchers develop potentially low-cost, low-emissions technology that can convert methane without forming CO2». Phys-Org. American Institute of Physics. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Freyermuth, George H. «1934 Patent: «The manufacture of hydrogen from methane hydrocarbons by the action of steam at elevated temperature»«. Patent Full-Text Databases. United States Patent and Trademark Office. Archived from the original on 1 October 2021. Retrieved 30 October 2020.
- ^ Press, Roman J.; Santhanam, K. S. V.; Miri, Massoud J.; Bailey, Alla V.; Takacs, Gerald A. (2008). Introduction to hydrogen Technology. John Wiley & Sons. p. 249. ISBN 978-0-471-77985-8.
- ^ a b c Oxtoby, D. W. (2002). Principles of Modern Chemistry (5th ed.). Thomson Brooks/Cole. ISBN 978-0-03-035373-4.
- ^ «Hydrogen Properties, Uses, Applications». Universal Industrial Gases, Inc. 2007. Archived from the original on 27 March 2008. Retrieved 11 March 2008.
- ^ Funderburg, E. (2008). «Why Are Nitrogen Prices So High?». The Samuel Roberts Noble Foundation. Archived from the original on 9 May 2001. Retrieved 11 March 2008.
- ^ Lees, A. (2007). «Chemicals from salt». BBC. Archived from the original on 26 October 2007. Retrieved 11 March 2008.
- ^ Parmuzina, A.V.; Kravchenko, O.V. (2008). «Activation of aluminium metal to evolve hydrogen from water». International Journal of Hydrogen Energy. 33 (12): 3073–3076. doi:10.1016/j.ijhydene.2008.02.025.
- ^ Weimer, Al (25 May 2005). «Development of solar-powered thermochemical production of hydrogen from water» (PDF). Solar Thermochemical Hydrogen Generation Project. Archived (PDF) from the original on 17 April 2007. Retrieved 21 December 2008.
- ^ Perret, R. «Development of Solar-Powered Thermochemical Production of Hydrogen from Water, DOE Hydrogen Program, 2007» (PDF). Archived from the original (PDF) on 27 May 2010. Retrieved 17 May 2008.
- ^ Russell, M. J.; Hall, A. J.; Martin, W. (2010). «Serpentinization as a source of energy at the origin of life». Geobiology. 8 (5): 355–371. doi:10.1111/j.1472-4669.2010.00249.x. PMID 20572872. S2CID 41118603.
- ^ Schrenk, M. O.; Brazelton, W. J.; Lang, S. Q. (2013). «Serpentinization, Carbon, and Deep Life» (PDF). Reviews in Mineralogy and Geochemistry. 75 (1): 575–606. Bibcode:2013RvMG…75..575S. doi:10.2138/rmg.2013.75.18. S2CID 8600635.
- ^ Smil, Vaclav (2004). Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (1st ed.). Cambridge, MA: MIT. ISBN 978-0-262-69313-4.
- ^ Chemistry Operations (15 December 2003). «Hydrogen». Los Alamos National Laboratory. Archived from the original on 4 March 2011. Retrieved 5 February 2008.
- ^ «DOE Seeks Applicants for Solicitation on the Employment Effects of a Transition to a Hydrogen Economy». Hydrogen Program (Press release). US Department of Energy. 22 March 2006. Archived from the original on 19 July 2011. Retrieved 16 March 2008.
- ^ McCarthy, J. (31 December 1995). «Hydrogen». Stanford University. Archived from the original on 14 March 2008. Retrieved 14 March 2008.
- ^ Reed, Stanley; Ewing, Jack (13 July 2021). «Hydrogen Is One Answer to Climate Change. Getting It Is the Hard Part». The New York Times. ISSN 0362-4331. Archived from the original on 14 July 2021. Retrieved 14 July 2021.
- ^ IRENA (2019). Hydrogen: A renewable energy perspective (PDF). p. 9. ISBN 978-92-9260-151-5. Archived (PDF) from the original on 29 September 2021. Retrieved 17 October 2021..
- ^ Bonheure, Mike; Vandewalle, Laurien A.; Marin, Guy B.; Van Geem, Kevin M. (March 2021). «Dream or Reality? Electrification of the Chemical Process Industries». CEP Magazine. American Institute of Chemical Engineers. Archived from the original on 17 July 2021. Retrieved 6 July 2021.
- ^ a b Griffiths, Steve; Sovacool, Benjamin K.; Kim, Jinsoo; Bazilian, Morgan; et al. (2021). «Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options». Energy Research & Social Science. 80: 39. doi:10.1016/j.erss.2021.102208. ISSN 2214-6296. Archived from the original on 16 October 2021. Retrieved 11 September 2021.
- ^ a b Evans, Simon; Gabbatiss, Josh (30 November 2020). «In-depth Q&A: Does the world need hydrogen to solve climate change?». Carbon Brief. Archived from the original on 1 December 2020. Retrieved 1 December 2020.
- ^ Palys, Matthew J.; Daoutidis, Prodromos (2020). «Using hydrogen and ammonia for renewable energy storage: A geographically comprehensive techno-economic study». Computers & Chemical Engineering. 136: 106785. doi:10.1016/j.compchemeng.2020.106785. ISSN 0098-1354.
- ^ IRENA (2021). World Energy Transitions Outlook: 1.5°C Pathway (PDF). pp. 12, 22. ISBN 978-92-9260-334-2. Archived (PDF) from the original on 11 June 2021.
- ^ IEA (2021). Net Zero by 2050: A Roadmap for the Global Energy Sector (PDF). pp. 15, 75–76. Archived (PDF) from the original on 23 May 2021.
- ^ Blank, Thomas; Molly, Patrick (January 2020). «Hydrogen’s Decarbonization Impact for Industry» (PDF). Rocky Mountain Institute. pp. 2, 7, 8. Archived (PDF) from the original on 22 September 2020.
- ^ Heffel, J. W. (2002). «NOx emission and performance data for a hydrogen fueled internal combustion engine at 1500 rpm using exhaust gas recirculation». International Journal of Hydrogen Energy. 28 (8): 901–908. doi:10.1016/S0360-3199(02)00157-X.
- ^ «Carbon Capture Strategy Could Lead to Emission-Free Cars» (Press release). Georgia Tech. 11 February 2008. Archived from the original on 28 September 2013. Retrieved 16 March 2008.
- ^ Romm, J. J. (2004). The Hype About Hydrogen: Fact And Fiction in the Race To Save The Climate (1st ed.). Island Press. ISBN 978-1-55963-703-9.
- ^ Le Comber, P. G.; Jones, D. I.; Spear, W. E. (1977). «Hall effect and impurity conduction in substitutionally doped amorphous silicon». Philosophical Magazine. 35 (5): 1173–1187. Bibcode:1977PMag…35.1173C. doi:10.1080/14786437708232943.
- ^ Van de Walle, C. G. (2000). «Hydrogen as a cause of doping in zinc oxide» (PDF). Physical Review Letters. 85 (5): 1012–1015. Bibcode:2000PhRvL..85.1012V. doi:10.1103/PhysRevLett.85.1012. hdl:11858/00-001M-0000-0026-D0E6-E. PMID 10991462. Archived (PDF) from the original on 15 August 2017. Retrieved 1 August 2018.
- ^ Janotti, A.; Van De Walle, C. G. (2007). «Hydrogen multicentre bonds». Nature Materials. 6 (1): 44–47. Bibcode:2007NatMa…6…44J. doi:10.1038/nmat1795. PMID 17143265.
- ^ Kilic, C.; Zunger, Alex (2002). «n-type doping of oxides by hydrogen». Applied Physics Letters. 81 (1): 73–75. Bibcode:2002ApPhL..81…73K. doi:10.1063/1.1482783. S2CID 96415065. Archived from the original on 29 January 2021. Retrieved 16 December 2019.
- ^ Peacock, P. W.; Robertson, J. (2003). «Behavior of hydrogen in high dielectric constant oxide gate insulators». Applied Physics Letters. 83 (10): 2025–2027. Bibcode:2003ApPhL..83.2025P. doi:10.1063/1.1609245.
- ^ Durgutlu, A. (2003). «Experimental investigation of the effect of hydrogen in argon as a shielding gas on TIG welding of austenitic stainless steel». Materials & Design. 25 (1): 19–23. doi:10.1016/j.matdes.2003.07.004.
- ^ «Atomic Hydrogen Welding». Specialty Welds. 2007. Archived from the original on 16 July 2011.
- ^ Hardy, W. N. (2003). «From H2 to cryogenic H masers to HiTc superconductors: An unlikely but rewarding path». Physica C: Superconductivity. 388–389: 1–6. Bibcode:2003PhyC..388….1H. doi:10.1016/S0921-4534(02)02591-1.
- ^ Almqvist, Ebbe (2003). History of industrial gases. New York, N.Y.: Kluwer Academic/Plenum Publishers. pp. 47–56. ISBN 978-0-306-47277-0. Retrieved 20 May 2015.
- ^ Block, M. (3 September 2004). Hydrogen as Tracer Gas for Leak Detection. 16th WCNDT 2004. Montreal, Canada: Sensistor Technologies. Archived from the original on 8 January 2009. Retrieved 25 March 2008.
- ^ «Report from the Commission on Dietary Food Additive Intake» (PDF). European Union. Archived (PDF) from the original on 16 February 2008. Retrieved 5 February 2008.
- ^ Reinsch, J.; Katz, A.; Wean, J.; Aprahamian, G.; MacFarland, J. T. (1980). «The deuterium isotope effect upon the reaction of fatty acyl-CoA dehydrogenase and butyryl-CoA». J. Biol. Chem. 255 (19): 9093–97. doi:10.1016/S0021-9258(19)70531-6. PMID 7410413.
- ^ «NASA/TM—2002-211915: Solid Hydrogen Experiments for Atomic Propellants» (PDF). Archived (PDF) from the original on 9 July 2021. Retrieved 2 July 2021.
- ^ Bergeron, K. D. (2004). «The Death of no-dual-use». Bulletin of the Atomic Scientists. 60 (1): 15–17. Bibcode:2004BuAtS..60a..15B. doi:10.2968/060001004. Archived from the original on 19 April 2008. Retrieved 13 April 2008.
- ^ Cammack, R.; Robson, R. L. (2001). Hydrogen as a Fuel: Learning from Nature. Taylor & Francis Ltd. pp. 202–203. ISBN 978-0-415-24242-4. Archived from the original on 29 January 2021. Retrieved 3 September 2020.
- ^ Rhee, T. S.; Brenninkmeijer, C. A. M.; Röckmann, T. (19 May 2006). «The overwhelming role of soils in the global atmospheric hydrogen cycle» (PDF). Atmospheric Chemistry and Physics. 6 (6): 1611–1625. Bibcode:2006ACP…..6.1611R. doi:10.5194/acp-6-1611-2006. Archived (PDF) from the original on 24 August 2019. Retrieved 24 August 2019.
- ^ Eisenmann, Alexander; Amann, Anton; Said, Michael; Datta, Bettina; Ledochowski, Maximilian (2008). «Implementation and interpretation of hydrogen breath tests» (PDF). Journal of Breath Research. 2 (4): 046002. Bibcode:2008JBR…..2d6002E. doi:10.1088/1752-7155/2/4/046002. PMID 21386189. S2CID 31706721. Archived from the original (PDF) on 29 January 2021. Retrieved 26 December 2020.
- ^ Berger, W. H. (15 November 2007). «The Future of Methane». University of California, San Diego. Archived from the original on 24 April 2008. Retrieved 12 February 2008.
- ^ Kruse, O.; Rupprecht, J.; Bader, K.; Thomas-Hall, S.; Schenk, P. M.; Finazzi, G.; Hankamer, B. (2005). «Improved photobiological H2 production in engineered green algal cells» (PDF). The Journal of Biological Chemistry. 280 (40): 34170–7. doi:10.1074/jbc.M503840200. PMID 16100118. S2CID 5373909. Archived (PDF) from the original on 29 January 2021. Retrieved 24 August 2019.
- ^ Smith, Hamilton O.; Xu, Qing (2005). «IV.E.6 Hydrogen from Water in a Novel Recombinant Oxygen-Tolerant Cyanobacteria System» (PDF). FY2005 Progress Report. United States Department of Energy. Archived (PDF) from the original on 29 December 2016. Retrieved 6 August 2016.
- ^ Williams, C. (24 February 2006). «Pond life: the future of energy». Science. The Register. Archived from the original on 9 May 2011. Retrieved 24 March 2008.
- ^ «MyChem: Chemical» (PDF). Archived from the original (PDF) on 1 October 2018. Retrieved 1 October 2018.
- ^ a b Brown, W. J.; et al. (1997). «Safety Standard for Hydrogen and Hydrogen Systems» (PDF). NASA. NSS 1740.16. Archived (PDF) from the original on 1 May 2017. Retrieved 12 July 2017.
- ^ «Liquid Hydrogen MSDS» (PDF). Praxair, Inc. September 2004. Archived from the original (PDF) on 27 May 2008. Retrieved 16 April 2008.
- ^ «‘Bugs’ and hydrogen embrittlement». Science News. 128 (3): 41. 20 July 1985. doi:10.2307/3970088. JSTOR 3970088.
- ^ Hayes, B. «Union Oil Amine Absorber Tower». TWI. Archived from the original on 20 November 2008. Retrieved 29 January 2010.
- ^ Walker, James L.; Waltrip, John S.; Zanker, Adam (1988). «Lactic acid to magnesium supply-demand relationships». In John J. McKetta; William Aaron Cunningham (eds.). Encyclopedia of Chemical Processing and Design. Vol. 28. New York: Dekker. p. 186. ISBN 978-0-8247-2478-8. Retrieved 20 May 2015.
Further reading
- Chart of the Nuclides (17th ed.). Knolls Atomic Power Laboratory. 2010. ISBN 978-0-9843653-0-2.
- Ferreira-Aparicio, P.; Benito, M. J.; Sanz, J. L. (2005). «New Trends in Reforming Technologies: from Hydrogen Industrial Plants to Multifuel Microreformers». Catalysis Reviews. 47 (4): 491–588. doi:10.1080/01614940500364958. S2CID 95966974.
- Newton, David E. (1994). The Chemical Elements. New York: Franklin Watts. ISBN 978-0-531-12501-4.
- Rigden, John S. (2002). Hydrogen: The Essential Element. Cambridge, Massachusetts: Harvard University Press. ISBN 978-0-531-12501-4.
- Romm, Joseph J. (2004). The Hype about Hydrogen, Fact and Fiction in the Race to Save the Climate. Island Press. ISBN 978-1-55963-703-9.
- Scerri, Eric (2007). The Periodic System, Its Story and Its Significance. New York: Oxford University Press. ISBN 978-0-19-530573-9.
- Hydrogen safety covers the safe production, handling and use
External links
![]()
These audio files were created from a revision of this article dated 28 October 2006, and do not reflect subsequent edits.
- Basic Hydrogen Calculations of Quantum Mechanics
- Hydrogen at The Periodic Table of Videos (University of Nottingham)
- High temperature hydrogen phase diagram
- Wavefunction of hydrogen
Положение в ПСХЭ водорода, лантаноидов, актиноидов и искусственно полученных элементов
I. Положение водорода в периодической системе
Водород – самый распространённый химический элемент, к тому же он самый лёгкий. Его порядковый номер 1. В таблице Менделеева он стоит в первом периоде. С учётом его свойств его помещают как в 1А так и в 7А группу. Возникает вопрос – почему?
Ядро водорода состоит из одного протона, вокруг которого вращается один электрон. Электронная формула 1s1. Молекула водорода состоит из двух атомов, связанных между собой ковалентной неполярной связью. Н2 – самый легкий газ. Он не имеет цвета и запаха.
Водород относится к химически активным веществам. Он может выступать в роли восстановителя и окислителя.
1) с некоторыми металлами он образует гидриды
2Na+H2=2NaH, здесь водород – окислительH0 + 1e— → H-1
Сходный процесс происходит при взаимодействии галогенов – неметаллов 7А группы
2Na+Cl2=2NaCl
Поэтому, водород помещают в 7А группу
2) с неметаллами, проявляющими более сильные окислительные свойства, чем водород
H2+Cl2 = 2HCl здесь водород – восстановительH0 — 1e— → H+1
Сходный процесс происходит при взаимодействии щелочных металлов –металлов 1А группы
2К+Cl2=2КCl
Поэтому, водород помещают в 1А группу
ИЮПАК рекомендует размещать водород только в 1А группе.
II. Положение в периодической системе химических элементов Д. И. Менделеева лантаноидов и актиноидов
Учебный фильм: “Свойства лантаноидов и актиноидов”
В шестом периоде вслед за лантаном располагаются 14 элементов с порядковыми номерами 58-71, называемых лантаноидами(слово “лантаноиды” означает «подобные лантану», а “актиноиды” — «подобные актинию»). Иногда их называют лантанидами и актинидами, что означает следующие за лантаном; следующие за актинием). Лантаноиды помещены отдельно внизу таблицы, а в клетке звездочкой указано на последовательность их расположения в системе: La-Lu. Химические свойства лантаноидов очень сходны. Например, все они являются реакционно-способными металлами, реагируют с водой с образованием гидроксида и водорода. У лантана (Z= 57) один электрон поступает на 5d-подуровень, после чего заполнение этого подуровня приостанавливается, а начинает заполняться 4f-уровень, семь орбиталей которого могут быть заняты 14 электронами. Это происходит у атомов всех лантаноидов с Z = 58 — 71. Поскольку у этих элементов заполняется глубинный 4f-подуровеиь третьего снаружи уровня, они обладают весьма близкими химическими свойствами.
Из этого следует, что у лантаноидов сильно выражена горизонтальная аналогия.
В седьмом периоде 14 элементов с порядковыми номерами 90-103 составляют семейство актиноидов. Их также помещают отдельно — под лантаноидами, а в соответствующей клетке двумя звездочками указано на последовательность их расположения в системе: Ас-Lr. У актиния и актиноидов заполнение уровней электронами подобно лантану и лантаноидам. Однако в отличие от лантаноидов горизонтальная аналогия у актиноидов выражена слабо. Они в своих соединениях проявляют больше различных степеней окисления. Например, степень окисления актиния +3, а урана +3, +4, +5 и +6. Изучение химических свойств актиноидов крайне сложно вследствие неустойчивости их ядер.
Все актиноиды радиоактивны. Из актиноидов выделяют две пересекающиеся группы: «трансурановые элементы» — все следующие в таблице Менделеева за ураном элементы и «трансплутониевые элементы» — все следующие за плутонием. Обе группы не ограничиваются указанными рамками и при указании приставки «транс-» могут включать в себя следующие за лоуренсием элементы — резерфордий и т. д. Это обусловлено тем, что такие элементы синтезируются в чрезвычайно малых количествах. По сравнению с лантаноидами, которые (кроме прометия) обнаружены в природе в заметных количествах, актиноиды труднее синтезировать. Но есть и исключения, например, легче всех синтезировать или найти в природе уран и торий, затем следуют плутоний, америций, актиний, протактиний и нептуний.
III. Положение в периодической системе химических элементов Д. И. Менделеева искусственно полученных элементов
К 2008 г. известно 117 химических элементов (с порядковыми номерами с 1 по 116 и 118), из них 94 обнаружены в природе (некоторые — лишь в следовых количествах), остальные 23 получены искусственно в результате ядерных реакций (см. Приложения). Первые 112 элементов имеют постоянные названия, остальные — временные.
Водород в таблице менделеева занимает 1 место, в 1 периоде.
| Символ | H |
| Номер | 1 |
| Атомный вес | 1.0078400 |
| Латинское название | Hydrogenium |
| Русское название | Водород |
Как самостоятельно построить электронную конфигурацию? Ответ здесь
Электронная схема водорода
H: 1s1
Порядок заполнения оболочек атома водорода (H) электронами:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d →
5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
На подуровне ‘s’ может находиться до 2 электронов, на ‘s’ — до 6, на
‘d’ — до 10 и на ‘f’ до 14
Водород имеет 1 электрон,
заполним электронные оболочки в описанном выше порядке:
1 электрон на 1s-подуровне
Степень окисления водорода
Атомы водорода в соединениях имеют степени окисления 1, 0, -1.
Степень окисления — это условный заряд атома в соединении: связь в молекуле
между атомами основана на разделении электронов, таким образом, если у атома виртуально увеличивается
заряд, то степень окисления отрицательная (электроны несут отрицательный заряд), если заряд уменьшается,
то степень окисления положительная.
Ионы водорода
Валентность H
Атомы водорода в соединениях проявляют валентность I.
Валентность водорода характеризует способность атома H к образованию хмических связей.
Валентность следует из строения электронной оболочки атома, электроны, участвующие в образовании
химических соединений называются валентными электронами. Более обширное определение валентности это:
Число химических связей, которыми данный атом соединён с другими атомами
Валентность не имеет знака.
Квантовые числа H
Квантовые числа определяются последним электроном в конфигурации,
для атома H эти числа имеют значение N = 1, L = 0, Ml = 0, Ms = +½
Видео заполнения электронной конфигурации (gif):
Результат:

Энергия ионизации
Чем ближе электрон к центру атома — тем больше энергии необходимо, что бы его оторвать.
Энергия, затрачиваемая на отрыв электрона от атома называется энергией ионизации и обозначается Eo.
Если не указано иное, то энергия ионизации — это энергия отрыва первого электрона, также существуют энергии
ионизации для каждого последующего электрона.
Энергия ионизации H:
Eo = 1312 кДж/моль
— Что такое ион читайте в статье.
Перейти к другим элементам таблицы менделеева
Где H в таблице менделеева?
Таблица Менделеева
Скачать таблицу менделеева в хорошем качестве
| Водород | |
|---|---|
| Газ без цвета, запаха и вкуса | |
 Водород в разрядной трубке | |
| Название, символ, номер | Водород / Hydrogenium (H), 1 |
| Атомная масса (молярная масса) | [1,00784; 1,00811]а. е. м. (г/моль) |
| Электронная конфигурация | 1s1 |
| Радиус атома | 53 пм |
| Ковалентный радиус | 32 пм |
| Радиус иона | 54 (−1 e) пм |
| Электроотрицательность | 2,20 (шкала Полинга) |
| Степени окисления | +1, 0, −1 |
| Энергия ионизации (первый электрон) | 1311,3 (13,595) кДж/моль (эВ) |
| Плотность (при н. у.) | 0,0000899 (при 273 K (0 °C)) г/см³ |
| Температура плавления | 14,01 K; −259,14 °C |
| Температура кипения | 20,28 K; −252,87 °C |
| Уд. теплота плавления | 0,117 кДж/моль |
| Уд. теплота испарения | 0,904 кДж/моль |
| Молярная теплоёмкость | 28,47 Дж/(K·моль) |
| Молярный объём | 14,1 см³/моль |
| Структура решётки | гексагональная |
| Параметры решётки | a = 3,780 c = 6,167 Å |
| Отношение c/a | 1,631 |
| Температура Дебая | 110 K |
| Теплопроводность | (300 K) 0,1815 Вт/(м·К) |
| Номер CAS | 12385-13-6 |
Водород (H, лат. hydrogenium) — химический элемент периодической системы с обозначением H и атомным номером 1, самый лёгкий из элементов периодической таблицы. Его одноатомная форма — самое распространённое химическое вещество во Вселенной, составляющее примерно 75 % всей барионной массы. Звёзды, кроме компактных, в основном состоят из водородной плазмы.
Три изотопа водорода имеют собственные названия: 1H — протий, 2H — дейтерий и 3H — тритий (радиоактивен). Ядро самого распространённого изотопа, протия, состоит из одного только протона и не содержит нейтронов.
При стандартных температуре и давлении водород — бесцветный, не имеющий запаха и вкуса, нетоксичный двухатомный газ с химической формулой H2, который в смеси с воздухом или кислородом горюч и крайне пожаро- и взрывоопасен. В присутствии других окисляющих газов, например фтора или хлора, водород также взрывоопасен. Поскольку водород охотно формирует ковалентные связи с большинством неметаллов, большая часть водорода на Земле существует в молекулярных соединениях, таких как вода или органические вещества. Водород играет особенно важную роль в кислотно-основных реакциях.
Растворим в этаноле и ряде металлов: железе, никеле, палладии, титане, платине, ниобии.
Содержание
- 1 История открытия
- 2 Происхождение названия
- 3 Распространённость
- 3.1 Во Вселенной
- 3.2 Земная кора и живые организмы
- 4 Получение
- 4.1 В промышленности
- 4.2 В лаборатории
- 4.3 Очистка
- 4.4 Стоимость
- 5 Физические свойства
- 6 Изотопы
- 6.1 Свойства изотопов
- 7 Химические свойства
- 7.1 Взаимодействие со щелочными и щёлочноземельными металлами
- 7.2 Взаимодействие с оксидами металлов
- 7.3 Гидрирование органических соединений
- 8 Геохимия водорода
- 9 Меры предосторожности
- 10 Применение
- 10.1 Химическая промышленность
- 10.2 Нефтеперерабатывающая промышленность
- 10.3 Пищевая и косметическая промышленность
- 10.4 Химические лаборатории
- 10.5 Авиационная промышленность
- 10.6 Метеорология
- 10.7 Топливо
- 10.8 Электроэнергетика
- 10.9 Прочее
История открытия
Выделение горючего газа при взаимодействии кислот и металлов наблюдали в XVI и XVII веках на заре становления химии как науки. Впервые водород получил Парацельс, погружая железные опилки в серную кислоту в XVI веке.
В 1671 году Роберт Бойль подробно описал реакцию между железными опилками и разбавленными кислотами, при которой выделяется газообразный водород.
В 1766 году Генри Кавендиш был первым, кто признал газообразный водород индивидуальным элементом, назвав газ, выделяющийся при реакции металла с кислотой «горючим воздухом». Он предположил, что «горючий воздух» идентичен гипотетическому веществу, называемому «флогистон», и в 1781 году обнаружил, что при его сгорании образуется вода.
Прямо указывал на выделение водорода и Михаил Ломоносов, но он уже понимал, что это не флогистон.
Французский химик Антуан Лавуазье совместно с инженером Жаном Мёнье, используя специальные газометры, в 1783 году осуществил синтез воды, а затем и её анализ, разложив водяной пар раскалённым железом. Так он установил, что «горючий воздух» входит в состав воды и может быть из неё получен.
Происхождение названия
Лавуазье дал водороду название hydrogène (от др.-греч. ὕδωρ — вода и γεννάω — рождаю) — «рождающий воду». В 1801 году последователь Лавуазье, академик Василий Севергин, называл его «водотворное вещество», он писал:
Водотворное вещество в соединении с кислотворным составляет воду. Сие можно доказать, как через разрешение, так и через составление.
Русское наименование «водород» предложил химик Михаил Соловьёв в 1824 году — по аналогии с «кислородом» Ломоносова.
Распространённость
Во Вселенной
Распространение ионизированного водорода в межзвёздной среде в различных частях нашей Галактики. Изображение в диапазоне H-альфа.
В настоящее время водород — самый распространённый элемент во Вселенной. На его долю приходится около 88,6 % всех атомов (около 11,3 % составляют атомы гелия, доля всех остальных вместе взятых элементов — порядка 0,1 %). Таким образом, водород — основная составная часть звёзд и межзвёздного газа. Повсеместное возникновение атомарного водорода впервые произошло в эпоху рекомбинации.
В условиях звёздных температур (например, температура поверхности Солнца ~6000 °C) водород существует в виде плазмы, в межзвёздном пространстве этот элемент существует в виде отдельных молекул, атомов и ионов и может образовывать молекулярные облака, значительно различающиеся по размерам, плотности и температуре.
Земная кора и живые организмы
Массовая доля водорода в земной коре составляет 1 % — это десятый по распространённости элемент. Однако его роль в природе определяется не массой, а числом атомов, доля которых среди остальных элементов составляет 17 % (второе место после кислорода, доля атомов которого равна ~52 %). Поэтому значение водорода в химических процессах, происходящих на Земле, почти так же велико, как и кислорода.
В отличие от кислорода, существующего на Земле и в связанном, и в свободном состояниях, практически весь водород на Земле находится в виде соединений; лишь в очень незначительном количестве водород в виде простого вещества содержится в атмосфере (0,00005 % по объёму для сухого воздуха).
Водород входит в состав практически всех органических веществ и присутствует во всех живых клетках, где по числу атомов на водород приходится почти 63 %.
Получение
Основная статья: Производство водорода
См. также: Биотехнологическое получение водорода
В промышленности
На 2019 год в мире потребляется 75 млн тонн водорода, в основном в нефтепереработке и производстве аммиака. Из них более 3/4 производится из природного газа, для чего расходуется более 205 млрд м3 газа. Почти все остальное получают из угля. Около 0,1 % (~100 тыс. тонн) вырабатывается электролизом. При производстве водорода в атмосферу поступает ~830 млн тонн CO2. Себестоимость водорода из природного газа оценивается в 1,5-3 доллара за 1 кг.
- Конверсия метана с водяным паром при 1000 °C:
-
- CH4 + H2O ⇄ CO + 3H2
- Пропускание паров воды над раскалённым коксом при температуре около 1000 °C:
-
- H2O + C ⇄ CO↑ + H2↑
- Электролиз водных растворов солей:
-
- 2NaCl + 2H2O → 2NaOH + Cl2↑ + H2↑
- Электролиз водных растворов гидроксидов активных металлов (преимущественно, гидроксида калия)
-
- 2H2O →4e− 2H2↑ + O2↑
- Кроме того, существует промышленная технология электролиза химически чистой воды, без применения каких-либо добавок. Фактически, устройство представляет собой обратимый топливный элемент с твёрдой полимерной мембраной.
- Каталитическое окисление кислородом:
-
- 2CH4 + O2 ⇄ 2CO + 4H2
- Крекинг и риформинг углеводородов в процессе переработки нефти.
В лаборатории
- Действие разбавленных кислот на металлы. Для проведения такой реакции чаще всего используют цинк и разбавленную серную кислоту:
-
- Zn + H2SO4 → ZnSO4 + H2↑
- Взаимодействие кальция с водой:
-
- Ca + 2H2O → Ca(OH)2 + H2↑
- Гидролиз гидридов:
-
- NaH + H2O → NaOH + H2↑
- Действие щелочей на цинк или алюминий:
-
- 2Al + 2NaOH + 6H2O → 2Na[Al(OH)4] + 3H2↑
-
- Zn + 2KOH + 2H2O → K2[Zn(OH)4] + H2↑
- С помощью электролиза. При электролизе водных растворов щелочей или кислот на катоде происходит выделение водорода, например:
-
- 2H3O+ + 2e− → 2H2O + H2↑
Очистка
В промышленности реализованы несколько способов очистки водорода из углерод-содержащего сырья (т. н. водородсодержащий газ — ВСГ).
- Низкотемпературная конденсация: ВСГ охлаждают до температур конденсации метана и этана, после чего водород отделяют ректификацией. Процесс ведут при температуре −158 °C и давлении 4 МПа. Чистота очищенного водорода составляет 93—94 % при его концентрации в исходном ВСГ до 40 %.
- Адсорбционное выделение на цеолитах: Настоящий метод на сегодняшний день наиболее распространён в мире. Метод достаточно гибок и может использоваться как для выделения водорода из ВСГ, так и для доочистки уже очищенного водорода. В первом случае процесс ведут при давлениях 3,0—3,5 МПа. Степень извлечения водорода составляет 80-85 % с чистотой 99 %. Во втором случае часто используют процесс «PSA» фирмы «Union Carbide». Он впервые был реализован в промышленности в 1978 году. На настоящий момент функционирует более 250 установок от 0,6 до 3,0 млн м3 H2/сут. Образуется водород высокой чистоты 99,99 %.
- Абсорбционное выделение жидкими растворителями: Этот метод применяется редко, хотя водород получается высокой чистоты 99,9 %.
- Концентрирование водорода на мембранах: На лучших образцах метод позволяет получать водород чистотой 95-96 %, однако производительность таких установок невысока.
- Селективное поглощение водорода металлами: Метод основан на способности сплавов лантана с никелем, железа с титаном, циркония с никелем и других поглощать до 30 объёмов водорода.
Стоимость
Стоимость водорода при крупнооптовых поставках колеблется в диапазоне 2—7 USD/кг. В небольших количествах перевозится в стальных баллонах зелёного или тёмно-зелёного цвета.
Физические свойства
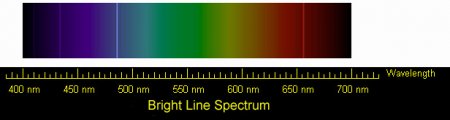
Эмиссионный спектр излучения атомов водорода на фоне сплошного спектра в видимой области

Эмиссионный спектр атомов водорода. Четыре видимые глазом спектральные линии серии Бальмера
Водород — самый лёгкий газ: он легче воздуха в 14,5 раз. Поэтому, например, мыльные пузыри, наполненные водородом, на воздухе стремятся вверх. Чем меньше масса молекул, тем выше их скорость при одной и той же температуре. Как самые лёгкие, молекулы водорода движутся быстрее молекул любого другого газа и тем самым быстрее могут передавать теплоту от одного тела к другому. Отсюда следует, что водород обладает самой высокой теплопроводностью среди газообразных веществ. Его теплопроводность примерно в 7 раз выше теплопроводности воздуха.
Молекула водорода двухатомна — H2. При нормальных условиях это газ без цвета, запаха и вкуса. Плотность 0,08987 г/л (н. у.), температура кипения −252,76 °C, удельная теплота сгорания 120,9⋅106 Дж/кг, малорастворим в воде — 18,8 мл/л.
Водород хорошо растворим во многих металлах (Ni, Pt, Pd и др.), особенно в палладии (850 объёмов H2 на 1 объём Pd). С растворимостью водорода в металлах связана его способность диффундировать через них; диффузия через углеродистый сплав (например, сталь) иногда сопровождается разрушением сплава вследствие взаимодействия водорода с углеродом (так называемая декарбонизация). Практически не растворим в серебре.
Фазовая диаграмма водорода
Жидкий водород существует в очень узком интервале температур от −252,76 до −259,2 °C. Это бесцветная жидкость, очень лёгкая (плотность при −253 °C 0,0708 г/см³) и текучая (вязкость при −253 °C 13,8 сП). Критические параметры водорода очень низкие: температура −240,2 °C и давление 12,8 атм. Этим объясняются трудности при ожижении водорода. В жидком состоянии равновесный водород состоит из 99,79 % пара-H2, 0,21 % орто-H2.
Твёрдый водород, температура плавления −259,2 °C, плотность 0,0807 г/см³ (при −262 °C) — снегоподобная масса, кристаллы гексагональной сингонии, пространственная группа P6/mmc, параметры ячейки a = 0,378 нм и c = 0,6167 нм.
В 1935 году Уингер и Хунтингтон высказали предположение о том, что при давлении свыше 250 тысяч атм водород может перейти в металлическое состояние. Получение этого вещества в устойчивом состоянии открывало очень заманчивые перспективы его применения — ведь это был бы сверхлёгкий металл, компонент лёгкого и энергоёмкого ракетного топлива. В 2014 году было установлено, что при давлении порядка 1,5—2,0 млн атм водород начинает поглощать инфракрасное излучение, а это означает, что электронные оболочки молекул водорода поляризуются. Возможно, при ещё более высоких давлениях водород превратится в металл. В 2017 году появилось сообщение о возможном экспериментальном наблюдении перехода водорода в металлическое состояние под высоким давлением.
Молекулярный водород существует в двух спиновых формах (модификациях): ортоводород и параводород. Модификации немного различаются по физическим свойствам, оптическим спектрам, также по характеристикам рассеивания нейтронов. В молекуле ортоводорода o-H2 (т. пл. −259,10 °C, т. кип. −252,56 °C) спины ядер параллельны, а у параводорода p-H2 (т. пл. −259,32 °C, т. кип. −252,89 °C) — противоположно друг другу (антипараллельны). Равновесная смесь o-H2 и p-H2 при заданной температуре называется равновесный водород e-H2.
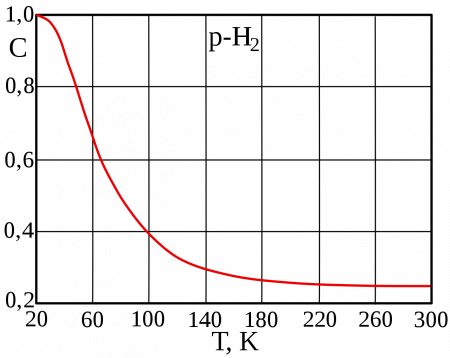
Равновесная мольная концентрация параводорода в смеси в зависимости от температуры
Разделить модификации водорода можно адсорбцией на активном угле при температуре жидкого азота. При очень низких температурах равновесие между ортоводородом и параводородом почти нацело сдвинуто в сторону параводорода, так как энергия пара-молекулы немного ниже энергии орто-молекулы. При 80 К соотношение модификаций приблизительно 1:1. Десорбированный с угля параводород при нагревании превращается в ортоводород с образованием равновесной смеси. При комнатной температуре равновесна смесь ортоводорода и параводорода в отношении около 75:25. Без катализатора взаимное превращение происходит относительно медленно, что даёт возможность изучить свойства обеих модификаций. В условиях разреженной межзвёздной среды характерное время перехода в равновесную смесь очень велико, вплоть до космологических.
Изотопы
Основная статья: Изотопы водорода
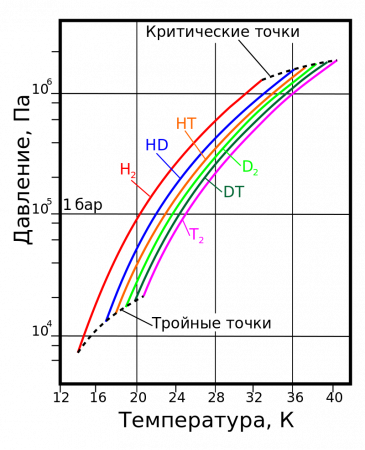
Термодинамическое состояние насыщенного пара водорода с различным изотопным составом
Наиболее известны три изотопа водорода: протий 1H (атомное ядро — протон), дейтерий 2H (ядро состоит из одного протона и одного нейтрона) и тритий 3H (ядро состоит из одного протона и двух нейтронов). Эти изотопы имеют собственные химические символы: протий — H, дейтерий — D, тритий — T.
Протий и дейтерий стабильны. Содержание этих изотопов в природном водороде составляет 99,9885 ± 0,0070 % и 0,0115 ± 0,0070 % соответственно. Оно может незначительно меняться в зависимости от источника и способа получения водорода. Тритий нестабилен, претерпевает бета-распад с периодом 12,32 года, превращаясь в стабильный гелий-3. Тритий встречается в природе в следовых количествах, образуясь главным образом при взаимодействии космических лучей со стабильными ядрами, при захвате дейтерием тепловых нейтронов и при взаимодействии природного изотопа лития-6 с нейтронами, порождёнными космическими лучами.
Искусственно получены также тяжёлые радиоактивные изотопы водорода с массовыми числами 4—7 и периодами полураспада 10−21—10−23 с.
Природный молекулярный водород состоит из молекул H2 и HD (дейтероводород) в соотношении 3200:1. Содержание в нём молекул из чистого дейтерия D2 ещё меньше, отношение концентраций HD и D2 составляет примерно 6400:1.
Из всех изотопов химических элементов физические свойства изотопов водорода отличаются друг от друга наиболее сильно. Это связано с наибольшим относительным изменением масс атомов.
-
Температура
плавления,
KТемпература
кипения,
KТройная
точкаКритическая
точкаПлотность,
кг/м³T, K P, кПа T, K P, МПа жидкий газ H2 13,96 20,39 13,96 7,3 32,98 1,31 70,811 1,316 HD 16,65 22,13 16,6 12,8 35,91 1,48 114,0 1,802 HT 22,92 17,63 17,7 37,13 1,57 158,62 2,31 D2 18,65 23,67 18,73 17,1 38,35 1,67 162,50 2,23 DT 24.38 19,71 19,4 39,42 1,77 211,54 2,694 T2 20,63 25,04 20,62 21,6 40,44 1,85 260,17 3,136
Молекулы чистых протия, дейтерия и трития могут существовать в двух аллотропных модификациях (отличающихся взаимной ориентацией спинов ядер) — орто- и параводород: o-D2, p-D2, o-T2, p-T2. Молекулы водорода с другим изотопным составом (HD, HT, DT) не имеют орто- и парамодификаций.
Свойства изотопов
Свойства изотопов водорода представлены в таблице.
| Изотоп | Z | N | Масса, а. е. м. | Период полураспада | Спин | Содержание в природе, % | Тип и энергия распада | |
|---|---|---|---|---|---|---|---|---|
| 1H | 1 | 0 | 1,007 825 032 07(10) | стабилен | 1⁄2+ | 99,9885(70) | ||
| 2H | 1 | 1 | 2,014 101 777 8(4) | стабилен | 1+ | 0,0115(70) | ||
| 3H | 1 | 2 | 3,016 049 277 7(25) | 12,32(2) года | 1⁄2+ | β− | 18,591(1) кэВ | |
| 4H | 1 | 3 | 4,027 81(11) | 1,39(10)⋅10−22 с | 2− | -n | 23,48(10) МэВ | |
| 5H | 1 | 4 | 5,035 31(11) | более 9,1⋅10−22 с | (1⁄2+) | -nn | 21,51(11) МэВ | |
| 6H | 1 | 5 | 6,044 94(28) | 2,90(70)⋅10−22 с | 2− | −3n | 24,27(26) МэВ | |
| 7H | 1 | 6 | 7,052 75(108) | 2,3(6)⋅10−23 с | 1⁄2+ | -nn | 23,03(101) МэВ |
В круглых скобках приведено среднеквадратическое отклонение значения в единицах последнего разряда соответствующего числа.
Свойства ядра 1H позволяют широко использовать ЯМР-спектроскопию в анализе органических веществ.
Химические свойства
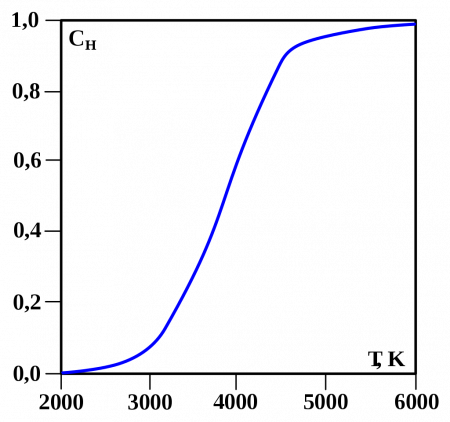
Доля диссоциировавших молекул водорода при атмосферном давлении в зависимости от температуры
Молекулы водорода достаточно прочны, и для того, чтобы водород мог вступить в реакцию, должна быть затрачена большая энергия:
-
- H2 → 2H− 432 кДж
Поэтому при обычных температурах водород реагирует только с очень активными металлами, например, с кальцием, образуя гидрид кальция:
-
- Ca + H2 → CaH2
и с единственным неметаллом — фтором, образуя фтороводород:
-
- F2 + H2 → 2HF
С большинством же металлов и неметаллов водород реагирует при повышенной температуре или при другом воздействии, например, при освещении:
-
- O2 + 2H2 → 2H2O
Записанное уравнение отражает восстановительные свойства водорода.
-
- CuO + H2 → H2O + Cu
С галогенами образует галогеноводороды:
-
- H2 + F2 → 2HF , реакция протекает со взрывом в темноте и при любой температуре,
-
- H2 + Cl2 → 2HCl , реакция протекает со взрывом, только на свету.
С сажей взаимодействует при сильном нагревании:
-
- C + 2H2 → CH4
Взаимодействие со щелочными и щёлочноземельными металлами
При взаимодействии с активными металлами водород образует гидриды:
-
- 2Na + H2 → 2NaH
-
- Ca + H2 → CaH2
-
- Mg + H2 → MgH2
Гидриды — солеобразные, твёрдые вещества, легко гидролизуются:
-
- CaH2 + 2H2O → Ca(OH)2 + 2H2↑
Взаимодействие с оксидами металлов
Оксиды металлов (как правило, d-элементов) восстанавливаются до металлов:
-
- Fe2O3 + 3H2 → 2Fe + 3H2O
-
- WO3 + 3H2 → W + 3H2O
Гидрирование органических соединений
Молекулярный водород широко применяется в органическом синтезе для восстановления органических соединений. Эти процессы называют реакциями гидрирования. Эти реакции проводят в присутствии катализатора при повышенных давлении и температуре. Катализатор может быть как гомогенным (напр., Катализатор Уилкинсона), так и гетерогенным (напр., никель Ренея, палладий на угле).
Так, в частности, при каталитическом гидрировании ненасыщенных соединений, таких как алкены и алкины, образуются насыщенные соединения — алканы.
R−CH = CH−R′ + H2 → R−CH2−CH2−R′
Геохимия водорода
На Земле содержание водорода понижено по сравнению с Солнцем, планетами-гигантами и первичными метеоритами, из чего следует, что во время образования Земля была значительно дегазирована: основная масса водорода, как и других летучих элементов, покинула планету во время аккреции или вскоре после неё. Однако точное содержание данного газа в составе геосфер нашей планеты (исключая земную кору) — астеносферы, мантии, ядра Земли — неизвестно.
Свободный водород H2 относительно редко встречается в земных газах, но в виде воды он принимает исключительно важное участие в геохимических процессах. Известно содержание водорода в составе вулканических газов, истечение некоторых количеств водорода вдоль разломов в зонах рифтогенеза, выделение этого газа в некоторых угольных месторождениях.
В состав минералов водород может входить в виде иона аммония, гидроксил-иона и воды.
В атмосфере молекулярный водород непрерывно образуется в результате разложения формальдегида, образующегося в цепочке окисления метана или другой органики, солнечным излучением (31—67 гигатонн/год), неполного сгорания различных топлив и биомасс (по 5—25 гигатонн/год), в процессе фиксации азота микроорганизмами из воздуха (3−22 гигатонн/год).
Имея малую массу, молекулы водорода в составе воздуха обладают высокой тепловой скоростью (она близка ко второй космической скорости) и, попадая в верхние слои атмосферы, могут навсегда улететь в космическое пространство (см. Диссипация атмосфер планет). Объёмы потерь оцениваются в 3 кг в секунду.
Меры предосторожности
Водород при смеси с воздухом образует взрывоопасную смесь — так называемый гремучий газ. Наибольшую взрывоопасность этот газ имеет при объёмном отношении водорода и кислорода 2:1, или водорода и воздуха приближённо 2:5, так как в воздухе кислорода содержится примерно 21 %. Также водород пожароопасен. Жидкий водород при попадании на кожу может вызвать сильное обморожение.
Считается, что взрывоопасные концентрации водорода с кислородом возникают от 4 % до 96 % объёмных. При смеси с воздухом от 4 % до 75 (74) % по объёму. Такие цифры фигурируют сейчас в большинстве справочников, и ими вполне можно пользоваться для ориентировочных оценок. Однако следует иметь в виду, что более поздние исследования (примерно конец 80-х) выявили, что водород в больших объёмах может быть взрывоопасен и при меньшей концентрации. Чем больше объём, тем меньшая концентрация водорода опасна.
Источник этой широко растиражированной ошибки в том, что взрывоопасность исследовалась в лабораториях на малых объёмах. Поскольку реакция водорода с кислородом — это цепная химическая реакция, которая проходит по свободнорадикальному механизму, «гибель» свободных радикалов на стенках (или, скажем, поверхности пылинок) критична для продолжения цепочки. В случаях, когда возможно создание «пограничных» концентраций в больших объёмах (помещения, ангары, цеха), следует иметь в виду, что реально взрывоопасная концентрация может отличаться от 4 % как в большую, так и в меньшую стороны.
Применение
Водород сегодня применяется во многих областях. Структура мирового потребления водорода представлена в следующей таблице
| Применение | Доля |
|---|---|
| Производство аммиака | 54 % |
| Нефтепереработка и химическая промышленность | 35 % |
| Производство электроники | 6 % |
| Металлургия и стекольная промышленность | 3 % |
| Пищевая промышленность | 2 % |
Химическая промышленность
Химическая промышленность — это крупнейший потребитель водорода. Около 50 % мирового выпуска водорода идёт на производство аммиака. Ещё около 8 % используется для производства метанола. Из аммиака производят пластмассы, удобрения, взрывчатые вещества и прочее. Метанол является основой для производства некоторых пластмасс.
Нефтеперерабатывающая промышленность
В нефтепереработке водород используется в процессах гидрокрекинга и гидроочистки, способствуя увеличению глубины переработки сырой нефти и повышению качества конечных продуктов. Для этих целей используется порядка 37 % всего производимого в мире водорода.
Пищевая и косметическая промышленность
При производстве саломаса (твёрдый жир, производимый из растительных масел). Саломас является основой для производства маргарина, косметических средств, мыла. Водород зарегистрирован в качестве пищевой добавки E949.
Химические лаборатории
Водород используется в химических лабораториях в качестве газа-носителя в газовой хроматографии. Такие лаборатории есть на многих предприятиях в пищевой, парфюмерной, металлургической и химической промышленности. Несмотря на горючесть водорода, его использование в такой роли считается достаточно безопасным, поскольку водород используется в незначительных количествах. Эффективность водорода как газа-носителя при этом лучше, чем у гелия, при существенно более низкой стоимости.
Авиационная промышленность
В настоящее время водород в авиации не используется. Когда-то дирижабли и воздушные шары наполняли водородом. Но в 30-х гг. XX в. произошло несколько катастроф, в ходе которых дирижабли взрывались и сгорали. В наше время дирижабли наполняют гелием, несмотря на его существенно более высокую стоимость.
Метеорология
Водород используется в метеорологии для заполнения оболочек метеозондов. Водород в этом качестве имеет преимущество перед гелием, так как он дешевле. Ещё более существенно, что водород вырабатывается прямо на метеостанции с помощью простого химического генератора или с помощью электролиза воды. Гелий же должен доставляться на метеостанцию в баллонах, что может быть затруднительно для удалённых мест.
Топливо
Водород используют в качестве ракетного топлива. Ввиду крайне узкого диапазона температур (менее 7 кельвинов), при котором водород остаётся жидкостью, на практике чаще используется смесь жидкой и твёрдой фаз .
Ведутся исследования по применению водорода как топлива для легковых и грузовых автомобилей.
В водородно-кислородных топливных элементах используется водород для непосредственного преобразования энергии химической реакции в электрическую.
Электроэнергетика
Водород применяется для охлаждения мощных электрических генераторов.
Прочее
Атомарный водород используется для атомно-водородной сварки. Высокая теплопроводность водорода используется для заполнения сфер гирокомпасов и стеклянных колб филаментных LED-лампочек.
| Периодическая система химических элементов Д. И. Менделеева | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ПЕРИОДИЧЕСКАЯ ТАБЛИЦА МЕНДЕЛЕЕВА
Еще в школе, сидя на уроках химии, все мы помним таблицу на стене класса или химической лаборатории. Эта таблица содержала классификацию всех известных человечеству химических элементов, тех фундаментальных компонентов, из которых состоит Земля и вся Вселенная. Тогда мы и подумать не могли, что таблица Менделеева бесспорно является одним из величайших научных открытий, который является фундаментом нашего современного знания о химии.
На первый взгляд, ее идея выглядит обманчиво просто: организовать химические элементы в порядке возрастания веса их атомов. Причем в большинстве случаев оказывается, что химические и физические свойства каждого элемента сходны с предыдущим ему в таблице элементом. Эта закономерность проявляется для всех элементов, кроме нескольких самых первых, просто потому что они не имеют перед собой элементов, сходных с ними по атомному весу. Именно благодаря открытию такого свойства мы можем поместить линейную последовательность элементов в таблицу, очень напоминающую настенный календарь, и таким образом объединить огромное количество видов химических элементов в четкой и связной форме. Разумеется, сегодня мы пользуемся понятием атомного числа (количества протонов) для того, чтобы упорядочить систему элементов. Это помогло решить так называемую техническую проблему «пары перестановок», однако не привело к кардинальному изменению вида периодической таблицы.
В периодической таблице Менделеева все элементы упорядочены с учетом их атомного числа, электронной конфигурации и повторяющихся химических свойств. Ряды в таблице называются периодами, а столбцы группами. В первой таблице, датируемой 1869 годом, содержалось всего 60 элементов, теперь же таблицу пришлось увеличить, чтобы поместить 118 элементов, известных нам сегодня.
Периодическая система Менделеева систематизирует не только элементы, но и самые разнообразные их свойства. Химику часто бывает достаточно иметь перед глазами Периодическую таблицу для того, чтобы правильно ответить на множество вопросов (не только экзаменационных, но и научных).
The YouTube ID of 1M7iKKVnPJE is invalid.
Периодический закон
Существуют две формулировки периодического закона химических элементов: классическая и современная.
Классическая, в изложении его первооткрывателя Д.И. Менделеева: свойства простых тел, а также формы и свойства соединений элементов находятся в периодической зависимости от величин атомных весов элементов.
Современная: свойства простых веществ, а также свойства и формы соединений элементов находятся в периодической зависимости от заряда ядра атомов элементов (порядкового номера).
Графическим изображением периодического закона является периодическая система элементов, которая представляет собой естественную классификацию химических элементов, основанную на закономерных изменениях свойств элементов от зарядов их атомов. Наиболее распространёнными изображениями периодической системы элементов Д.И. Менделеева являются короткая и длинная формы.

Группы и периоды Периодической системы
Группами называют вертикальные ряды в периодической системе. В группах элементы объединены по признаку высшей степени окисления в оксидах. Каждая группа состоит из главной и побочной подгрупп. Главные подгруппы включают в себя элементы малых периодов и одинаковые с ним по свойствам элементы больших периодов. Побочные подгруппы состоят только из элементов больших периодов. Химические свойства элементов главных и побочных подгрупп значительно различаются.
Периодом называют горизонтальный ряд элементов, расположенных в порядке возрастания порядковых (атомных) номеров. В периодической системе имеются семь периодов: первый, второй и третий периоды называют малыми, в них содержится соответственно 2, 8 и 8 элементов; остальные периоды называют большими: в четвёртом и пятом периодах расположены по 18 элементов, в шестом — 32, а в седьмом (пока незавершенном) — 31 элемент. Каждый период, кроме первого, начинается щелочным металлом, а заканчивается благородным газом.
Физический смысл порядкового номера химического элемента: число протонов в атомном ядре и число электронов, вращающихся вокруг атомного ядра, равны порядковому номеру элемента.
Свойства таблицы Менделеева
Напомним, что группами называют вертикальные ряды в периодической системе и химические свойства элементов главных и побочных подгрупп значительно различаются.
Свойства элементов в подгруппах закономерно изменяются сверху вниз:
- усиливаются металлические свойства и ослабевают неметаллические;
- возрастает атомный радиус;
- возрастает сила образованных элементом оснований и бескислородных кислот;
- электроотрицательность падает.
Все элементы, кроме гелия, неона и аргона, образуют кислородные соединения, существует всего восемь форм кислородных соединений. В периодической системе их часто изображают общими формулами, расположенными под каждой группой в порядке возрастания степени окисления элементов: R2O, RO, R2O3, RO2, R2O5, RO3, R2O7, RO4, где символом R обозначают элемент данной группы. Формулы высших оксидов относятся ко всем элементам группы, кроме исключительных случаев, когда элементы не проявляют степени окисления, равной номеру группы (например, фтор).
Оксиды состава R2O проявляют сильные основные свойства, причём их основность возрастает с увеличением порядкового номера, оксиды состава RO (за исключением BeO) проявляют основные свойства. Оксиды состава RO2, R2O5, RO3, R2O7 проявляют кислотные свойства, причём их кислотность возрастает с увеличением порядкового номера.
Элементы главных подгрупп, начиная с IV группы, образуют газообразные водородные соединения. Существуют четыре формы таких соединений. Их располагают под элементами главных подгрупп и изображают общими формулами в последовательности RH4, RH3, RH2, RH.
Соединения RH4 имеют нейтральный характер; RH3 — слабоосновный; RH2 — слабокислый; RH — сильнокислый характер.
Напомним, что периодом называют горизонтальный ряд элементов, расположенных в порядке возрастания порядковых (атомных) номеров.
В пределах периода с увеличением порядкового номера элемента:
- электроотрицательность возрастает;
- металлические свойства убывают, неметаллические возрастают;
- атомный радиус падает.
Элементы таблицы Менделеева
Щелочные и щелочноземельные элементы
К ним относятся элементы из первой и второй группы периодической таблицы. Щелочные металлы из первой группы — мягкие металлы, серебристого цвета, хорошо режутся ножом. Все они обладают одним-единственным электроном на внешней оболочке и прекрасно вступают в реакцию. Щелочноземельные металлы из второй группы также имеют серебристый оттенок. На внешнем уровне помещено по два электрона, и, соответственно, эти металлы менее охотно взаимодействуют с другими элементами. По сравнению со щелочными металлами, щелочноземельные металлы плавятся и кипят при более высоких температурах.
Показать / Скрыть текст
| Щелочные металлы | Щелочноземельные металлы |
| Литий Li 3 | Бериллий Be 4 |
| Натрий Na 11 | Магний Mg 12 |
| Калий K 19 | Кальций Ca 20 |
| Рубидий Rb 37 | Стронций Sr 38 |
| Цезий Cs 55 | Барий Ba 56 |
| Франций Fr 87 | Радий Ra 88 |
Лантаниды (редкоземельные элементы) и актиниды
Лантаниды — это группа элементов, изначально обнаруженных в редко встречающихся минералах; отсюда их название «редкоземельные» элементы. Впоследствии выяснилось, что данные элементы не столь редки, как думали вначале, и поэтому редкоземельным элементам было присвоено название лантаниды. Лантаниды и актиниды занимают два блока, которые расположены под основной таблицей элементов. Обе группы включают в себя металлы; все лантаниды (за исключением прометия) нерадиоактивны; актиниды, напротив, радиоактивны.
Показать / Скрыть текст
| Лантаниды | Актиниды |
| Лантан La 57 | Актиний Ac 89 |
| Церий Ce 58 | Торий Th 90 |
| Празеодимий Pr 59 | Протактиний Pa 91 |
| Неодимий Nd 60 | Уран U 92 |
| Прометий Pm 61 | Нептуний Np 93 |
| Самарий Sm 62 | Плутоний Pu 94 |
| Европий Eu 63 | Америций Am 95 |
| Гадолиний Gd 64 | Кюрий Cm 96 |
| Тербий Tb 65 | Берклий Bk 97 |
| Диспрозий Dy 66 | Калифорний Cf 98 |
| Гольмий Ho 67 | Эйнштейний Es 99 |
| Эрбий Er 68 | Фермий Fm 100 |
| Тулий Tm 69 | Менделевий Md 101 |
| Иттербий Yb 70 | Нобелий No 102 |
Галогены и благородные газы
Галогены и благородные газы объединены в группы 17 и 18 периодической таблицы. Галогены представляют собой неметаллические элементы, все они имеют семь электронов во внешней оболочке. В благородных газахвсе электроны находятся во внешней оболочке, таким образом с трудом участвуют в образовании соединений. Эти газы называют «благородными, потому что они редко вступают в реакцию с прочими элементами; т. е. ссылаются на представителей благородной касты, которые традиционно сторонились других людей в обществе.
Показать / Скрыть текст
| Галогены | Благородные газы |
| Фтор F 9 | Гелий He 2 |
| Хлор Cl 17 | Неон Ne 10 |
| Бром Br 35 | Аргон Ar 18 |
| Йод I 53 | Криптон Kr 36 |
| Астат At 85 | Ксенон Xe 54 |
| — | Радон Rn 86 |
Переходные металлы
Переходные металлы занимают группы 3—12 в периодической таблице. Большинство из них плотные, твердые, с хорошей электро- и теплопроводностью. Их валентные электроны (при помощи которых они соединяются с другими элементами) находятся в нескольких электронных оболочках.
Показать / Скрыть текст
| Переходные металлы |
| Скандий Sc 21 |
| Титан Ti 22 |
| Ванадий V 23 |
| Хром Cr 24 |
| Марганец Mn 25 |
| Железо Fe 26 |
| Кобальт Co 27 |
| Никель Ni 28 |
| Медь Cu 29 |
| Цинк Zn 30 |
| Иттрий Y 39 |
| Цирконий Zr 40 |
| Ниобий Nb 41 |
| Молибден Mo 42 |
| Технеций Tc 43 |
| Рутений Ru 44 |
| Родий Rh 45 |
| Палладий Pd 46 |
| Серебро Ag 47 |
| Кадмий Cd 48 |
| Лютеций Lu 71 |
| Гафний Hf 72 |
| Тантал Ta 73 |
| Вольфрам W 74 |
| Рений Re 75 |
| Осмий Os 76 |
| Иридий Ir 77 |
| Платина Pt 78 |
| Золото Au 79 |
| Ртуть Hg 80 |
| Лоуренсий Lr 103 |
| Резерфордий Rf 104 |
| Дубний Db 105 |
| Сиборгий Sg 106 |
| Борий Bh 107 |
| Хассий Hs 108 |
| Мейтнерий Mt 109 |
| Дармштадтий Ds 110 |
| Рентгений Rg 111 |
| Коперниций Cn 112 |
Металлоиды
Металлоиды занимают группы 13—16 периодической таблицы. Такие металлоиды, как бор, германий и кремний, являются полупроводниками и используются для изготовления компьютерных чипов и плат.
Показать / Скрыть текст
| Металлоиды |
| Бор B 5 |
| Кремний Si 14 |
| Германий Ge 32 |
| Мышьяк As 33 |
| Сурьма Sb 51 |
| Теллур Te 52 |
| Полоний Po 84 |
Постпереходными металлами
Элементы, называемые постпереходными металлами, относятся к группам 13—15 периодической таблицы. В отличие от металлов, они не имеют блеска, а имеют матовую окраску. В сравнении с переходными металлами постпереходные металлы более мягкие, имеют более низкую температуру плавления и кипения, более высокую электроотрицательность. Их валентные электроны, с помощью которых они присоединяют другие элементы, располагаются только на внешней электронной оболочке. Элементы группы постпереходных металлов имеют гораздо более высокую температуру кипения, чем металлоиды.
Показать / Скрыть текст
| Постпереходные металлы |
| Алюминий Al 13 |
| Галлий Ga 31 |
| Индий In 49 |
| Олово Sn 50 |
| Таллий Tl 81 |
| Свинец Pb 82 |
| Висмут Bi 83 |
Неметаллы
Из всех элементов, классифицируемых как неметаллы, водород относится к 1-й группе периодической таблицы, а остальные — к группам 13—18. Неметаллы не являются хорошими проводниками тепла и электричества. Обычно при комнатной температуре они пребывают в газообразном (водород или кислород) или твердом состоянии (углерод).
Показать / Скрыть текст
| Неметаллы |
| Водород H 1 |
| Углерод C 6 |
| Азот N 7 |
| Кислород O 8 |
| Фосфор P 15 |
| Сера S 16 |
| Селен Se 34 |
| Флеровий Fl 114 |
| Унунсептий Uus 117 |
А теперь закрепите полученные знания, посмотрев видео про таблицу Менделеева и не только.
Отлично, первый шаг на пути к знаниям сделан. Теперь вы более-менее ориентируетесь в таблице Менделеева и это вам очень даже пригодится, ведь Периодическая система Менделеева является фундаментом, на котором стоит эта удивительная наука.


