Not to be confused with Iridium.
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | (IN-dee-əm) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery lustrous gray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(In) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 49 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 13 (boron group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 429.7485 K (156.5985 °C, 313.8773 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2345 K (2072 °C, 3762 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.31 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 7.02 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 429.7445 K, ~1 kPa[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 3.281 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 231.8 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.74 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −5, −2, −1, 0,[3] +1, +2, +3[4] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.78 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 167 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 142±5 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 193 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of indium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered tetragonal
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 1215 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 32.1 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 81.8 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 83.7 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −64.0×10−6 cm3/mol (298 K)[6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 11 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 8.8–10.0 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-74-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Ferdinand Reich and Hieronymous Theodor Richter (1863) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Hieronymous Theodor Richter (1864) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of indium
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth’s crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties.[7] Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year.
Indium is a minor component in zinc sulfide ores and is produced as a byproduct of zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conductive coatings of indium tin oxide (ITO) on glass. Indium is considered a technology-critical element.
Indium has no biological role. Its compounds are toxic when injected into the bloodstream. Most occupational exposure is through ingestion, from which indium compounds are not absorbed well, and inhalation, from which they are moderately absorbed.
Properties[edit]
Physical[edit]

Indium wetting the glass surface of a test tube
Indium is a silvery-white, highly ductile post-transition metal with a bright luster.[8] It is so soft (Mohs hardness 1.2) that like sodium, it can be cut with a knife. It also leaves a visible line on paper.[9] It is a member of group 13 on the periodic table and its properties are mostly intermediate between its vertical neighbours gallium and thallium. Like tin, a high-pitched cry is heard when indium is bent – a crackling sound due to crystal twinning.[8] Like gallium, indium is able to wet glass. Like both, indium has a low melting point, 156.60 °C (313.88 °F); higher than its lighter homologue, gallium, but lower than its heavier homologue, thallium, and lower than tin.[10] The boiling point is 2072 °C (3762 °F), higher than that of thallium, but lower than gallium, conversely to the general trend of melting points, but similarly to the trends down the other post-transition metal groups because of the weakness of the metallic bonding with few electrons delocalized.[11]
The density of indium, 7.31 g/cm3, is also greater than gallium, but lower than thallium. Below the critical temperature, 3.41 K, indium becomes a superconductor. Indium crystallizes in the body-centered tetragonal crystal system in the space group I4/mmm (lattice parameters: a = 325 pm, c = 495 pm):[10] this is a slightly distorted face-centered cubic structure, where each indium atom has four neighbours at 324 pm distance and eight neighbours slightly further (336 pm).[12] Indium has greater solubility in liquid mercury than any other metal (more than 50 mass percent of indium at 0 °C).[13] Indium displays a ductile viscoplastic response, found to be size-independent in tension and compression. However it does have a size effect in bending and indentation, associated to a length-scale of order 50–100 µm,[14] significantly large when compared with other metals.
Chemical[edit]
Indium has 49 electrons, with an electronic configuration of [Kr]4d105s25p1. In compounds, indium most commonly donates the three outermost electrons to become indium(III), In3+. In some cases, the pair of 5s-electrons are not donated, resulting in indium(I), In+. The stabilization of the monovalent state is attributed to the inert pair effect, in which relativistic effects stabilize the 5s-orbital, observed in heavier elements. Thallium (indium’s heavier homolog) shows an even stronger effect, causing oxidation to thallium(I) to be more probable than to thallium(III),[15] whereas gallium (indium’s lighter homolog) commonly shows only the +3 oxidation state. Thus, although thallium(III) is a moderately strong oxidizing agent, indium(III) is not, and many indium(I) compounds are powerful reducing agents.[16] While the energy required to include the s-electrons in chemical bonding is lowest for indium among the group 13 metals, bond energies decrease down the group so that by indium, the energy released in forming two additional bonds and attaining the +3 state is not always enough to outweigh the energy needed to involve the 5s-electrons.[17] Indium(I) oxide and hydroxide are more basic and indium(III) oxide and hydroxide are more acidic.[17]
A number of standard electrode potentials, depending on the reaction under study,[18] are reported for indium, reflecting the decreased stability of the +3 oxidation state:[12]
-
In2+ + e− ⇌ In+ E0 = −0.40 V In3+ + e− ⇌ In2+ E0 = −0.49 V In3+ + 2 e− ⇌ In+ E0 = −0.443 V In3+ + 3 e− ⇌ In E0 = −0.3382 V In+ + e− ⇌ In E0 = −0.14 V
Indium metal does not react with water, but it is oxidized by stronger oxidizing agents such as halogens to give indium(III) compounds. It does not form a boride, silicide, or carbide, and the hydride InH3 has at best a transitory existence in ethereal solutions at low temperatures, being unstable enough to spontaneously polymerize without coordination.[16] Indium is rather basic in aqueous solution, showing only slight amphoteric characteristics, and unlike its lighter homologs aluminium and gallium, it is insoluble in aqueous alkaline solutions.[19]
Isotopes[edit]
Indium has 39 known isotopes, ranging in mass number from 97 to 135. Only two isotopes occur naturally as primordial nuclides: indium-113, the only stable isotope, and indium-115, which has a half-life of 4.41×1014 years, four orders of magnitude greater than the age of the Universe and nearly 30,000 times greater than that of natural thorium.[20] The half-life of 115In is very long because the beta decay to 115Sn is spin-forbidden.[21] Indium-115 makes up 95.7% of all indium. Indium is one of three known elements (the others being tellurium and rhenium) of which the stable isotope is less abundant in nature than the long-lived primordial radioisotopes.[22]
The stablest artificial isotope is indium-111, with a half-life of approximately 2.8 days. All other isotopes have half-lives shorter than 5 hours. Indium also has 47 meta states, among which indium-114m1 (half-life about 49.51 days) is the most stable, more stable than the ground state of any indium isotope other than the primordial. All decay by isomeric transition. The indium isotopes lighter than 115In predominantly decay through electron capture or positron emission to form cadmium isotopes, while the other indium isotopes from 115In and greater predominantly decay through beta-minus decay to form tin isotopes.[20]
Compounds[edit]
Indium(III)[edit]
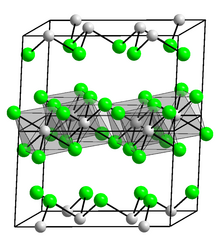
InCl3 (structure pictured) is a common compound of indium.
Indium(III) oxide, In2O3, forms when indium metal is burned in air or when the hydroxide or nitrate is heated.[23] In2O3 adopts a structure like alumina and is amphoteric, that is able to react with both acids and bases. Indium reacts with water to reproduce soluble indium(III) hydroxide, which is also amphoteric; with alkalis to produce indates(III); and with acids to produce indium(III) salts:
- In(OH)3 + 3 HCl → InCl3 + 3 H2O
The analogous sesquichalcogenides with sulfur, selenium, and tellurium are also known.[24] Indium forms the expected trihalides. Chlorination, bromination, and iodination of In produce colorless InCl3, InBr3, and yellow InI3. The compounds are Lewis acids, somewhat akin to the better known aluminium trihalides. Again like the related aluminium compound, InF3 is polymeric.[25]
Direct reaction of indium with the pnictogens produces the gray or semimetallic III–V semiconductors. Many of them slowly decompose in moist air, necessitating careful storage of semiconductor compounds to prevent contact with the atmosphere. Indium nitride is readily attacked by acids and alkalis.[26]
Indium(I)[edit]
Indium(I) compounds are not common. The chloride, bromide, and iodide are deeply colored, unlike the parent trihalides from which they are prepared. The fluoride is known only as an unstable gaseous compound.[27] Indium(I) oxide black powder is produced when indium(III) oxide decomposes upon heating to 700 °C.[23]
Other oxidation states[edit]
Less frequently, indium forms compounds in oxidation state +2 and even fractional oxidation states. Usually such materials feature In–In bonding, most notably in the halides In2X4 and [In2X6]2−,[28] and various subchalcogenides such as In4Se3.[29] Several other compounds are known to combine indium(I) and indium(III), such as InI6(InIIICl6)Cl3,[30] InI5(InIIIBr4)2(InIIIBr6),[31] and InIInIIIBr4.[28]
Organoindium compounds[edit]
Organoindium compounds feature In–C bonds. Most are In(III) derivatives, but cyclopentadienylindium(I) is an exception. It was the first known organoindium(I) compound,[32] and is polymeric, consisting of zigzag chains of alternating indium atoms and cyclopentadienyl complexes.[33] Perhaps the best-known organoindium compound is trimethylindium, In(CH3)3, used to prepare certain semiconducting materials.[34][35]
History[edit]
In 1863, the German chemists Ferdinand Reich and Hieronymous Theodor Richter were testing ores from the mines around Freiberg, Saxony. They dissolved the minerals pyrite, arsenopyrite, galena and sphalerite in hydrochloric acid and distilled raw zinc chloride. Reich, who was color-blind, employed Richter as an assistant for detecting the colored spectral lines. Knowing that ores from that region sometimes contain thallium, they searched for the green thallium emission spectrum lines. Instead, they found a bright blue line. Because that blue line did not match any known element, they hypothesized a new element was present in the minerals. They named the element indium, from the indigo color seen in its spectrum, after the Latin indicum, meaning ‘of India’.[36][37][38][39]
Richter went on to isolate the metal in 1864.[40] An ingot of 0.5 kg (1.1 lb) was presented at the World Fair 1867.[41] Reich and Richter later fell out when the latter claimed to be the sole discoverer.[39]
Occurrence[edit]

Indium is created by the long-lasting (up to thousands of years) s-process (slow neutron capture) in low-to-medium-mass stars (range in mass between 0.6 and 10 solar masses). When a silver-109 atom captures a neutron, it transmutes into silver-110, which then undergoes beta decay to become cadmium-110. Capturing further neutrons, it becomes cadmium-115, which decays to indium-115 by another beta decay. This explains why the radioactive isotope is more abundant than the stable one.[42] The stable indium isotope, indium-113, is one of the p-nuclei, the origin of which is not fully understood; although indium-113 is known to be made directly in the s- and r-processes (rapid neutron capture), and also as the daughter of very long-lived cadmium-113, which has a half-life of about eight quadrillion years, this cannot account for all indium-113.[43][44]
Indium is the 68th most abundant element in Earth’s crust at approximately 50 ppb. This is similar to the crustal abundance of silver, bismuth and mercury. It very rarely forms its own minerals, or occurs in elemental form. Fewer than 10 indium minerals such as roquesite (CuInS2) are known, and none occur at sufficient concentrations for economic extraction.[45] Instead, indium is usually a trace constituent of more common ore minerals, such as sphalerite and chalcopyrite.[46][47] From these, it can be extracted as a by-product during smelting.[48] While the enrichment of indium in these deposits is high relative to its crustal abundance, it is insufficient, at current prices, to support extraction of indium as the main product.[45]
Different estimates exist of the amounts of indium contained within the ores of other metals.[49][50] However, these amounts are not extractable without mining of the host materials (see Production and availability). Thus, the availability of indium is fundamentally determined by the rate at which these ores are extracted, and not their absolute amount. This is an aspect that is often forgotten in the current debate, e.g. by the Graedel group at Yale in their criticality assessments,[51] explaining the paradoxically low depletion times some studies cite.[52][48]
Production and availability[edit]

World production trend[53]
Indium is produced exclusively as a by-product during the processing of the ores of other metals. Its main source material are sulfidic zinc ores, where it is mostly hosted by sphalerite.[48] Minor amounts are probably also extracted from sulfidic copper ores. During the roast-leach-electrowinning process of zinc smelting, indium accumulates in the iron-rich residues. From these, it can be extracted in different ways. It may also be recovered directly from the process solutions. Further purification is done by electrolysis.[54] The exact process varies with the mode of operation of the smelter.[8][48]
Its by-product status means that indium production is constrained by the amount of sulfidic zinc (and copper) ores extracted each year. Therefore, its availability needs to be discussed in terms of supply potential. The supply potential of a by-product is defined as that amount which is economically extractable from its host materials per year under current market conditions (i.e. technology and price).[55] Reserves and resources are not relevant for by-products, since they cannot be extracted independently from the main-products.[48] Recent estimates put the supply potential of indium at a minimum of 1,300 t/yr from sulfidic zinc ores and 20 t/yr from sulfidic copper ores.[48] These figures are significantly greater than current production (655 t in 2016).[56] Thus, major future increases in the by-product production of indium will be possible without significant increases in production costs or price. The average indium price in 2016 was US$240/kg, down from US$705/kg in 2014.[57]
China is a leading producer of indium (290 tonnes in 2016), followed by South Korea (195 t), Japan (70 t) and Canada (65 t).[56] The Teck Resources refinery in Trail, British Columbia, is a large single-source indium producer, with an output of 32.5 tonnes in 2005, 41.8 tonnes in 2004 and 36.1 tonnes in 2003.
The primary consumption of indium worldwide is LCD production. Demand rose rapidly from the late 1990s to 2010 with the popularity of LCD computer monitors and television sets, which now account for 50% of indium consumption.[58] Increased manufacturing efficiency and recycling (especially in Japan) maintain a balance between demand and supply. According to the UNEP, indium’s end-of-life recycling rate is less than 1%.[59]
Applications[edit]

A magnified image of an LCD screen showing RGB pixels. Individual transistors are seen as white dots in the bottom part.
In 1924, indium was found to have a valued property of stabilizing non-ferrous metals, and that became the first significant use for the element.[60] The first large-scale application for indium was coating bearings in high-performance aircraft engines during World War II, to protect against damage and corrosion; this is no longer a major use of the element.[54] New uses were found in fusible alloys, solders, and electronics. In the 1950s, tiny beads of indium were used for the emitters and collectors of PNP alloy-junction transistors. In the middle and late 1980s, the development of indium phosphide semiconductors and indium tin oxide thin films for liquid-crystal displays (LCD) aroused much interest. By 1992, the thin-film application had become the largest end use.[61][62]
Indium(III) oxide and indium tin oxide (ITO) are used as a transparent conductive coating on glass substrates in electroluminescent panels.[63] Indium tin oxide is used as a light filter in low-pressure sodium-vapor lamps. The infrared radiation is reflected back into the lamp, which increases the temperature within the tube and improves the performance of the lamp.[62]
Indium has many semiconductor-related applications. Some indium compounds, such as indium antimonide and indium phosphide,[64] are semiconductors with useful properties: one precursor is usually trimethylindium (TMI), which is also used as the semiconductor dopant in II–VI compound semiconductors.[65] InAs and InSb are used for low-temperature transistors and InP for high-temperature transistors.[54] The compound semiconductors InGaN and InGaP are used in light-emitting diodes (LEDs) and laser diodes.[66] Indium is used in photovoltaics as the semiconductor copper indium gallium selenide (CIGS), also called CIGS solar cells, a type of second-generation thin-film solar cell.[67] Indium is used in PNP bipolar junction transistors with germanium: when soldered at low temperature, indium does not stress the germanium.[54]

A video on indium lung, an illness caused by indium exposure
Indium wire is used as a vacuum seal and a thermal conductor in cryogenics and ultra-high-vacuum applications, in such manufacturing applications as gaskets that deform to fill gaps.[68] Owing to its great plasticity and adhesion to metals, Indium sheets are sometimes used for cold-soldering in microwave circuits and waveguide joints, where direct soldering is complicated. Indium is an ingredient in the gallium–indium–tin alloy galinstan, which is liquid at room temperature and replaces mercury in some thermometers.[69] Other alloys of indium with bismuth, cadmium, lead, and tin, which have higher but still low melting points (between 50 and 100 °C), are used in fire sprinkler systems and heat regulators.[54]
Indium is one of many substitutes for mercury in alkaline batteries to prevent the zinc from corroding and releasing hydrogen gas.[70] Indium is added to some dental amalgam alloys to decrease the surface tension of the mercury and allow for less mercury and easier amalgamation.[71]
Indium’s high neutron-capture cross-section for thermal neutrons makes it suitable for use in control rods for nuclear reactors, typically in an alloy of 80% silver, 15% indium, and 5% cadmium.[72] In nuclear engineering, the (n,n’) reactions of 113In and 115In are used to determine magnitudes of neutron fluxes.[73]
In 2009, Professor Mas Subramanian and associates at Oregon State University discovered that indium can be combined with yttrium and manganese to form an intensely blue, non-toxic, inert, fade-resistant pigment, YInMn blue, the first new inorganic blue pigment discovered in 200 years.[74]
Biological role and precautions[edit]
| Hazards | |
|---|---|
| GHS labelling: | |
| Pictograms |  |
| Signal word | Warning |
| Hazard statements | H302, H312, H315, H319, H332, H335 |
| Precautionary statements | P261, P280, P305+P351+P338[75] |
| NFPA 704 (fire diamond) |
2 0 0 |
Indium has no metabolic role in any organism. In a similar way to aluminium salts, indium(III) ions can be toxic to the kidney when given by injection.[76] Indium tin oxide and indium phosphide harm the pulmonary and immune systems, predominantly through ionic indium,[77] though hydrated indium oxide is more than forty times as toxic when injected, measured by the quantity of indium introduced.[76] Radioactive indium-111 (in very small amounts on a chemical basis) is used in nuclear medicine tests, as a radiotracer to follow the movement of labeled proteins and white blood cells in the body.[78][79] Indium compounds are mostly not absorbed upon ingestion and are only moderately absorbed on inhalation; they tend to be stored temporarily in the muscles, skin, and bones before being excreted, and the biological half-life of indium is about two weeks in humans.[80]
People can be exposed to indium in the workplace by inhalation, ingestion, skin contact, and eye contact. Indium lung is a lung disease characterized by pulmonary alveolar proteinosis and pulmonary fibrosis, first described by Japanese researchers in 2003. As of 2010, 10 cases had been described, though more than 100 indium workers had documented respiratory abnormalities.[81] The National Institute for Occupational Safety and Health has set a recommended exposure limit (REL) of 0.1 mg/m3 over an eight-hour workday.[82]
See also[edit]
References[edit]
- ^ «Standard Atomic Weights: Indium». CIAAW. 2011.
- ^ Mangum, B. W. (1989). «Determination of the Indium Freezing-point and Triple-point Temperatures». Metrologia. 26 (4): 211. Bibcode:1989Metro..26..211M. doi:10.1088/0026-1394/26/4/001.
- ^ Unstable In(0) carbonyls and clusters have been detected, see [1], p. 6.
- ^ Guloy, A. M.; Corbett, J. D. (1996). «Synthesis, Structure, and Bonding of Two Lanthanum Indium Germanides with Novel Structures and Properties». Inorganic Chemistry. 35 (9): 2616–22. doi:10.1021/ic951378e. PMID 11666477.
- ^ Lide, D. R., ed. (2005). «Magnetic susceptibility of the elements and inorganic compounds». CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ W. M. Haynes (2010). David R. Lide (ed.). CRC Handbook of Chemistry and Physics: A Ready-reference Book of Chemical and Physical Data. CRC Press. ISBN 978-1-4398-2077-3.
- ^ a b c Alfantazi, A. M.; Moskalyk, R. R. (2003). «Processing of indium: a review». Minerals Engineering. 16 (8): 687–694. doi:10.1016/S0892-6875(03)00168-7.
- ^ Binder, Harry H. (1999). Lexicon der chemischen Elemente (in German). S. Hirzel Verlag. ISBN 978-3-7776-0736-8.
- ^ a b Dean, John A. (523). Lange’s handbook of chemistry (Fifteenth ed.). McGraw-Hill, Inc. ISBN 978-0-07-016190-0.
- ^ Greenwood and Earnshaw, p. 222
- ^ a b Greenwood and Earnshaw, p. 252
- ^ Okamoto, H. (2012). «Hg-In phase diagram». Journal of Phase Equilibria and Diffusion. 33 (2): 159–160. doi:10.1007/s11669-012-9993-3. S2CID 93043767.
- ^ Iliev, S. P.; Chen, X.; Pathan, M. V.; Tagarielli, V. L. (2017-01-23). «Measurements of the mechanical response of Indium and of its size dependence in bending and indentation». Materials Science and Engineering: A. 683: 244–251. doi:10.1016/j.msea.2016.12.017. hdl:10044/1/43082.
- ^ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). «Thallium». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 892–893. ISBN 978-3-11-007511-3.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Greenwood and Earnshaw, p. 256
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 8.20. ISBN 1-4398-5511-0.
- ^ Greenwood and Earnshaw, p. 255
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Dvornický, R.; Šimkovic, F. (13–16 June 2011). «Second unique forbidden β decay of 115In and neutrino mass». AIP Conf. Proc. AIP Conference Proceedings. 1417 (33): 33. Bibcode:2011AIPC.1417…33D. doi:10.1063/1.3671032.
- ^ «IUPAC Periodic Table of the Isotopes» (PDF). ciaaw.org. IUPAC. 1 October 2013. Retrieved 21 June 2016.
- ^ a b Anthony John Downs (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. ISBN 978-0-7514-0103-5.
- ^ Greenwood and Earnshaw, p. 286
- ^ Greenwood and Earnshaw, pp. 263–7
- ^ Greenwood and Earnshaw, p. 288
- ^ Greenwood and Earnshaw, pp. 270–1
- ^ a b Sinclair, Ian; Worrall, Ian J. (1982). «Neutral complexes of the indium dihalides». Canadian Journal of Chemistry. 60 (6): 695–698. doi:10.1139/v82-102.
- ^ Greenwood and Earnshaw, p. 287
- ^ Beck, Horst Philipp; Wilhelm, Doris (1991). «In7Cl9—A New»Old» Compound in the System In-Cl». Angewandte Chemie International Edition in English. 30 (7): 824–825. doi:10.1002/anie.199108241.
- ^ Dronskowski, Richard (1995). «Synthesis, Structure, and Decay of In4Br7». Angewandte Chemie International Edition in English. 34 (10): 1126–1128. doi:10.1002/anie.199511261.
- ^ Fischer, E. O.; Hofmann, H. P. (1957). «Metall-cyclopentadienyle des Indiums». Angewandte Chemie (in German). 69 (20): 639–640. Bibcode:1957AngCh..69..639F. doi:10.1002/ange.19570692008.
- ^ Beachley O. T.; Pazik J. C.; Glassman T. E.; Churchill M. R.; Fettinger J.C.; Blom R. (1988). «Synthesis, characterization and structural studies of In(C5H4Me) by x-ray diffraction and electron diffraction techniques and a reinvestigation of the crystalline state of In(C5H5) by x-ray diffraction studies». Organometallics. 7 (5): 1051–1059. doi:10.1021/om00095a007.
- ^ Shenai, Deo V.; Timmons, Michael L.; Dicarlo, Ronald L.; Lemnah, Gregory K.; Stennick, Robert S. (2003). «Correlation of vapor pressure equation and film properties with trimethylindium purity for the MOVPE grown III–V compounds». Journal of Crystal Growth. 248: 91–98. Bibcode:2003JCrGr.248…91S. doi:10.1016/S0022-0248(02)01854-7.
- ^ Shenai, Deodatta V.; Timmons, Michael L.; Dicarlo, Ronald L.; Marsman, Charles J. (2004). «Correlation of film properties and reduced impurity concentrations in sources for III/V-MOVPE using high-purity trimethylindium and tertiarybutylphosphine». Journal of Crystal Growth. 272 (1–4): 603–608. Bibcode:2004JCrGr.272..603S. doi:10.1016/j.jcrysgro.2004.09.006.
- ^ Reich, F.; Richter, T. (1863). «Ueber das Indium». Journal für Praktische Chemie (in German). 90 (1): 172–176. doi:10.1002/prac.18630900122. S2CID 94381243.
- ^ Venetskii, S. (1971). «Indium». Metallurgist. 15 (2): 148–150. doi:10.1007/BF01088126.
- ^ Greenwood and Earnshaw, p. 244
- ^ a b Weeks, Mary Elvira (1932). «The Discovery of the Elements: XIII. Some Spectroscopic Studies». Journal of Chemical Education. 9 (8): 1413–1434. Bibcode:1932JChEd…9.1413W. doi:10.1021/ed009p1413.[permanent dead link]
- ^ Reich, F.; Richter, T. (1864). «Ueber das Indium». Journal für Praktische Chemie (in German). 92 (1): 480–485. doi:10.1002/prac.18640920180.
- ^ Schwarz-Schampera, Ulrich; Herzig, Peter M. (2002). Indium: Geology, Mineralogy, and Economics. Springer. ISBN 978-3-540-43135-0.
- ^ Boothroyd, A. I. (2006). «Heavy elements in stars». Science. 314 (5806): 1690–1691. doi:10.1126/science.1136842. PMID 17170281. S2CID 116938510.
- ^ Arlandini, C.; Käppeler, F.; Wisshak, K.; Gallino, R.; Lugaro, M.; Busso, M.; Straniero, O. (1999). «Neutron Capture in Low-Mass Asymptotic Giant Branch Stars: Cross Sections and Abundance Signatures». The Astrophysical Journal. 525 (2): 886–900. arXiv:astro-ph/9906266. Bibcode:1999ApJ…525..886A. doi:10.1086/307938. S2CID 10847307.
- ^ Zs; Käppeler, F.; Theis, C.; Belgya, T.; Yates, S. W. (1994). «Nucleosynthesis in the Cd-In-Sn region». The Astrophysical Journal. 426: 357–365. Bibcode:1994ApJ…426..357N. doi:10.1086/174071.
- ^ a b Frenzel, Max (2016). «The distribution of gallium, germanium and indium in conventional and non-conventional resources — Implications for global availability (PDF Download Available)». ResearchGate. doi:10.13140/rg.2.2.20956.18564. Retrieved 2017-06-02.
- ^ Frenzel, Max; Hirsch, Tamino; Gutzmer, Jens (July 2016). «Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type — A meta-analysis». Ore Geology Reviews. 76: 52–78. doi:10.1016/j.oregeorev.2015.12.017.
- ^ Bachmann, Kai; Frenzel, Max; Krause, Joachim; Gutzmer, Jens (June 2017). «Advanced Identification and Quantification of In-Bearing Minerals by Scanning Electron Microscope-Based Image Analysis». Microscopy and Microanalysis. 23 (3): 527–537. Bibcode:2017MiMic..23..527B. doi:10.1017/S1431927617000460. ISSN 1431-9276. PMID 28464970. S2CID 6751828.
- ^ a b c d e f Frenzel, Max; Mikolajczak, Claire; Reuter, Markus A.; Gutzmer, Jens (June 2017). «Quantifying the relative availability of high-tech by-product metals – The cases of gallium, germanium and indium». Resources Policy. 52: 327–335. doi:10.1016/j.resourpol.2017.04.008.
- ^ «Mineral Commodities Summary 2007: Indium» (PDF). United States Geological Survey. Retrieved 2007-12-26.
- ^ Werner, T. T.; Mudd, G. M.; Jowitt, S. M. (2015-10-02). «Indium: key issues in assessing mineral resources and long-term supply from recycling». Applied Earth Science. 124 (4): 213–226. doi:10.1179/1743275815Y.0000000007. ISSN 0371-7453. S2CID 128555024.
- ^ Graedel, T. E.; Barr, Rachel; Chandler, Chelsea; Chase, Thomas; Choi, Joanne; Christoffersen, Lee; Friedlander, Elizabeth; Henly, Claire; Jun, Christine (2012-01-17). «Methodology of Metal Criticality Determination». Environmental Science & Technology. 46 (2): 1063–1070. Bibcode:2012EnST…46.1063G. doi:10.1021/es203534z. ISSN 0013-936X. PMID 22191617.
- ^ Harper, E. M.; Kavlak, Goksin; Burmeister, Lara; Eckelman, Matthew J.; Erbis, Serkan; Sebastian Espinoza, Vicente; Nuss, Philip; Graedel, T. E. (2015-08-01). «Criticality of the Geological Zinc, Tin, and Lead Family». Journal of Industrial Ecology. 19 (4): 628–644. doi:10.1111/jiec.12213. ISSN 1530-9290. S2CID 153380535.[permanent dead link]
- ^ U.S. Geological Survey – Historical Statistics for Mineral and Material Commodities in the United States; INDIUM STATISTICS // USGS, April 1, 2014
- ^ a b c d e Greenwood and Earnshaw, p. 247
- ^ Frenzel, Max; Tolosana-Delgado, Raimon; Gutzmer, Jens (December 2015). «Assessing the supply potential of high-tech metals – A general method». Resources Policy. 46, Part 2: 45–58. doi:10.1016/j.resourpol.2015.08.002.
- ^ a b Indium — in: USGS Mineral Commodity Summaries (PDF). United States Geological Survey. 2017.
- ^ Kelly, TD; Matos, GR (2015). «Historical Statistics for Mineral and Material Commodities in the United States». Retrieved 2017-06-02.
- ^ «Indium Price Supported by LCD Demand and New Uses for the Metal». Geology.com. Archived from the original (PDF) on 2007-12-21. Retrieved 2007-12-26.
- ^ «USGS Mineral Commodity Summaries 2011» (PDF). USGS and USDI. Retrieved August 2, 2011.
- ^ French, Sidney J. (1934). «A story of indium». Journal of Chemical Education. 11 (5): 270. Bibcode:1934JChEd..11..270F. doi:10.1021/ed011p270.
- ^ Tolcin, Amy C. «Mineral Yearbook 2007: Indium» (PDF). United States Geological Survey.
- ^ a b Downs, Anthony John (1993). Chemistry of Aluminium, Gallium, Indium, and Thallium. Springer. pp. 89 and 106. ISBN 978-0-7514-0103-5.
- ^ «The Electroluminescent Light Sabre». Nanotechnology News Archive. Azonano. June 2, 2005. Archived from the original on October 12, 2007. Retrieved 2007-08-29.
- ^ Bachmann, K. J. (1981). «Properties, Preparation, and Device Applications of Indium Phosphide». Annual Review of Materials Science. 11: 441–484. Bibcode:1981AnRMS..11..441B. doi:10.1146/annurev.ms.11.080181.002301.
- ^ Shenai, Deodatta V.; Timmons, Michael L.; DiCarlo Jr., Ronald L.; Marsman, Charles J. (2004). «Correlation of film properties and reduced impurity concentrations in sources for III/V-MOVPE using high-purity trimethylindium and tertiarybutylphosphine». Journal of Crystal Growth. 272 (1–4): 603–608. Bibcode:2004JCrGr.272..603S. doi:10.1016/j.jcrysgro.2004.09.006.
- ^ Schubert, E. Fred (2003). Light-Emitting Diodes. Cambridge University Press. p. 16. ISBN 978-0-521-53351-5.
- ^ Powalla, M.; Dimmler, B. (2000). «Scaling up issues of CIGS solar cells». Thin Solid Films. 361–362 (1–2): 540–546. Bibcode:2000TSF…361..540P. doi:10.1016/S0040-6090(99)00849-4.
- ^ Weissler, G. L., ed. (1990). Vacuum physics and technology. San Diego: Acad. Press. p. 296. ISBN 978-0-12-475914-5.
- ^ Surmann, P; Zeyat, H (Nov 2005). «Voltammetric analysis using a self-renewable non-mercury electrode». Analytical and Bioanalytical Chemistry. 383 (6): 1009–13. doi:10.1007/s00216-005-0069-7. PMID 16228199. S2CID 22732411.
- ^ Geological Survey (U.S.) (2010). Minerals Yearbook, 2008, V. 1, Metals and Minerals. Government Printing Office. pp. 35–2. ISBN 978-1-4113-3015-3.
- ^ Powell L. V., Johnson G. H., Bales D. J. (1989). «Effect of Admixed Indium on Mercury Vapor Release from Dental Amalgam». Journal of Dental Research. 68 (8): 1231–3. CiteSeerX 10.1.1.576.2654. doi:10.1177/00220345890680080301. PMID 2632609. S2CID 28342583.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Scoullos, Michael J. (2001-12-31). «Other types of cadmium alloys». Mercury, cadmium, lead: handbook for sustainable heavy metals policy and regulation. p. 222. ISBN 978-1-4020-0224-3.
- ^ Berger, Harold; National Bureau Of Standards, United States; Committee E-7 On Nondestructive Testing, American Society for Testing and Materials (1976). «Image Detectors for Other Neutron Energies». Practical applications of neutron radiography and gaging: a symposium. pp. 50–51.
- ^ Kupferschmidt, Kai (2019-05-02). «In search of blue». Science. American Association for the Advancement of Science (AAAS). 364 (6439): 424–429. Bibcode:2019Sci…364..424K. doi:10.1126/science.364.6439.424. ISSN 0036-8075. PMID 31048474. S2CID 143434096.
- ^ «Indium 57083».
- ^ a b Castronovo, F. P.; Wagner, H. N. (October 1971). «Factors Affecting the Toxicity of the Element Indium». British Journal of Experimental Pathology. 52 (5): 543–559. PMC 2072430. PMID 5125268.
- ^ Gwinn, W. M.; Qu, W.; Bousquet, R. W.; Price, H.; Shines, C. J.; Taylor, G. J.; Waalkes, M. P.; Morgan, D. L. (2014). «Macrophage Solubilization and Cytotoxicity of Indium-Containing Particles as in vitro Correlates to Pulmonary Toxicity in vivo». Toxicological Sciences. 144 (1): 17–26. doi:10.1093/toxsci/kfu273. PMC 4349143. PMID 25527823.
- ^ «IN-111 FACT SHEET» (PDF). Nordion(Canada), Inc. Archived from the original (PDF) on 3 December 2011. Retrieved 23 September 2012.
- ^ Van Nostrand, D.; Abreu, S. H.; Callaghan, J. J.; Atkins, F. B.; Stoops, H. C.; Savory, C. G. (May 1988). «In-111-labeled white blood cell uptake in noninfected closed fracture in humans: prospective study». Radiology. 167 (2): 495–498. doi:10.1148/radiology.167.2.3357961. PMID 3357961.
- ^ Nordberg, Gunnar F.; Fowler, Bruce A.; Nordberg, Monica (7 August 2014). Handbook on the Toxicology of Metals (4th ed.). Academic Press. p. 845. ISBN 978-0-12-397339-9.
- ^ Sauler, Maor; Gulati, Mridu (December 2012). «Newly Recognized Occupational and Environmental Causes of Chronic Terminal Airways and Parenchymal Lung Disease». Clinics in Chest Medicine. 33 (4): 667–680. doi:10.1016/j.ccm.2012.09.002. PMC 3515663. PMID 23153608.
- ^ «CDC – NIOSH Pocket Guide to Chemical Hazards – Indium». www.cdc.gov. Retrieved 2015-11-06.
Sources[edit]
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
External links[edit]
- Indium at The Periodic Table of Videos (University of Nottingham)
- Reducing Agents > Indium low valent
- NIOSH Pocket Guide to Chemical Hazards (Centers for Disease Control and Prevention)
Not to be confused with Iridium.
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | (IN-dee-əm) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery lustrous gray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(In) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 49 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 13 (boron group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 429.7485 K (156.5985 °C, 313.8773 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2345 K (2072 °C, 3762 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.31 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 7.02 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 429.7445 K, ~1 kPa[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 3.281 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 231.8 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.74 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −5, −2, −1, 0,[3] +1, +2, +3[4] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.78 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 167 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 142±5 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 193 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spectral lines of indium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered tetragonal
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 1215 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 32.1 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 81.8 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 83.7 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −64.0×10−6 cm3/mol (298 K)[6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 11 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 8.8–10.0 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-74-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Ferdinand Reich and Hieronymous Theodor Richter (1863) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Hieronymous Theodor Richter (1864) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Main isotopes of indium
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| references |
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth’s crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties.[7] Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year.
Indium is a minor component in zinc sulfide ores and is produced as a byproduct of zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conductive coatings of indium tin oxide (ITO) on glass. Indium is considered a technology-critical element.
Indium has no biological role. Its compounds are toxic when injected into the bloodstream. Most occupational exposure is through ingestion, from which indium compounds are not absorbed well, and inhalation, from which they are moderately absorbed.
Properties[edit]
Physical[edit]

Indium wetting the glass surface of a test tube
Indium is a silvery-white, highly ductile post-transition metal with a bright luster.[8] It is so soft (Mohs hardness 1.2) that like sodium, it can be cut with a knife. It also leaves a visible line on paper.[9] It is a member of group 13 on the periodic table and its properties are mostly intermediate between its vertical neighbours gallium and thallium. Like tin, a high-pitched cry is heard when indium is bent – a crackling sound due to crystal twinning.[8] Like gallium, indium is able to wet glass. Like both, indium has a low melting point, 156.60 °C (313.88 °F); higher than its lighter homologue, gallium, but lower than its heavier homologue, thallium, and lower than tin.[10] The boiling point is 2072 °C (3762 °F), higher than that of thallium, but lower than gallium, conversely to the general trend of melting points, but similarly to the trends down the other post-transition metal groups because of the weakness of the metallic bonding with few electrons delocalized.[11]
The density of indium, 7.31 g/cm3, is also greater than gallium, but lower than thallium. Below the critical temperature, 3.41 K, indium becomes a superconductor. Indium crystallizes in the body-centered tetragonal crystal system in the space group I4/mmm (lattice parameters: a = 325 pm, c = 495 pm):[10] this is a slightly distorted face-centered cubic structure, where each indium atom has four neighbours at 324 pm distance and eight neighbours slightly further (336 pm).[12] Indium has greater solubility in liquid mercury than any other metal (more than 50 mass percent of indium at 0 °C).[13] Indium displays a ductile viscoplastic response, found to be size-independent in tension and compression. However it does have a size effect in bending and indentation, associated to a length-scale of order 50–100 µm,[14] significantly large when compared with other metals.
Chemical[edit]
Indium has 49 electrons, with an electronic configuration of [Kr]4d105s25p1. In compounds, indium most commonly donates the three outermost electrons to become indium(III), In3+. In some cases, the pair of 5s-electrons are not donated, resulting in indium(I), In+. The stabilization of the monovalent state is attributed to the inert pair effect, in which relativistic effects stabilize the 5s-orbital, observed in heavier elements. Thallium (indium’s heavier homolog) shows an even stronger effect, causing oxidation to thallium(I) to be more probable than to thallium(III),[15] whereas gallium (indium’s lighter homolog) commonly shows only the +3 oxidation state. Thus, although thallium(III) is a moderately strong oxidizing agent, indium(III) is not, and many indium(I) compounds are powerful reducing agents.[16] While the energy required to include the s-electrons in chemical bonding is lowest for indium among the group 13 metals, bond energies decrease down the group so that by indium, the energy released in forming two additional bonds and attaining the +3 state is not always enough to outweigh the energy needed to involve the 5s-electrons.[17] Indium(I) oxide and hydroxide are more basic and indium(III) oxide and hydroxide are more acidic.[17]
A number of standard electrode potentials, depending on the reaction under study,[18] are reported for indium, reflecting the decreased stability of the +3 oxidation state:[12]
-
In2+ + e− ⇌ In+ E0 = −0.40 V In3+ + e− ⇌ In2+ E0 = −0.49 V In3+ + 2 e− ⇌ In+ E0 = −0.443 V In3+ + 3 e− ⇌ In E0 = −0.3382 V In+ + e− ⇌ In E0 = −0.14 V
Indium metal does not react with water, but it is oxidized by stronger oxidizing agents such as halogens to give indium(III) compounds. It does not form a boride, silicide, or carbide, and the hydride InH3 has at best a transitory existence in ethereal solutions at low temperatures, being unstable enough to spontaneously polymerize without coordination.[16] Indium is rather basic in aqueous solution, showing only slight amphoteric characteristics, and unlike its lighter homologs aluminium and gallium, it is insoluble in aqueous alkaline solutions.[19]
Isotopes[edit]
Indium has 39 known isotopes, ranging in mass number from 97 to 135. Only two isotopes occur naturally as primordial nuclides: indium-113, the only stable isotope, and indium-115, which has a half-life of 4.41×1014 years, four orders of magnitude greater than the age of the Universe and nearly 30,000 times greater than that of natural thorium.[20] The half-life of 115In is very long because the beta decay to 115Sn is spin-forbidden.[21] Indium-115 makes up 95.7% of all indium. Indium is one of three known elements (the others being tellurium and rhenium) of which the stable isotope is less abundant in nature than the long-lived primordial radioisotopes.[22]
The stablest artificial isotope is indium-111, with a half-life of approximately 2.8 days. All other isotopes have half-lives shorter than 5 hours. Indium also has 47 meta states, among which indium-114m1 (half-life about 49.51 days) is the most stable, more stable than the ground state of any indium isotope other than the primordial. All decay by isomeric transition. The indium isotopes lighter than 115In predominantly decay through electron capture or positron emission to form cadmium isotopes, while the other indium isotopes from 115In and greater predominantly decay through beta-minus decay to form tin isotopes.[20]
Compounds[edit]
Indium(III)[edit]

InCl3 (structure pictured) is a common compound of indium.
Indium(III) oxide, In2O3, forms when indium metal is burned in air or when the hydroxide or nitrate is heated.[23] In2O3 adopts a structure like alumina and is amphoteric, that is able to react with both acids and bases. Indium reacts with water to reproduce soluble indium(III) hydroxide, which is also amphoteric; with alkalis to produce indates(III); and with acids to produce indium(III) salts:
- In(OH)3 + 3 HCl → InCl3 + 3 H2O
The analogous sesquichalcogenides with sulfur, selenium, and tellurium are also known.[24] Indium forms the expected trihalides. Chlorination, bromination, and iodination of In produce colorless InCl3, InBr3, and yellow InI3. The compounds are Lewis acids, somewhat akin to the better known aluminium trihalides. Again like the related aluminium compound, InF3 is polymeric.[25]
Direct reaction of indium with the pnictogens produces the gray or semimetallic III–V semiconductors. Many of them slowly decompose in moist air, necessitating careful storage of semiconductor compounds to prevent contact with the atmosphere. Indium nitride is readily attacked by acids and alkalis.[26]
Indium(I)[edit]
Indium(I) compounds are not common. The chloride, bromide, and iodide are deeply colored, unlike the parent trihalides from which they are prepared. The fluoride is known only as an unstable gaseous compound.[27] Indium(I) oxide black powder is produced when indium(III) oxide decomposes upon heating to 700 °C.[23]
Other oxidation states[edit]
Less frequently, indium forms compounds in oxidation state +2 and even fractional oxidation states. Usually such materials feature In–In bonding, most notably in the halides In2X4 and [In2X6]2−,[28] and various subchalcogenides such as In4Se3.[29] Several other compounds are known to combine indium(I) and indium(III), such as InI6(InIIICl6)Cl3,[30] InI5(InIIIBr4)2(InIIIBr6),[31] and InIInIIIBr4.[28]
Organoindium compounds[edit]
Organoindium compounds feature In–C bonds. Most are In(III) derivatives, but cyclopentadienylindium(I) is an exception. It was the first known organoindium(I) compound,[32] and is polymeric, consisting of zigzag chains of alternating indium atoms and cyclopentadienyl complexes.[33] Perhaps the best-known organoindium compound is trimethylindium, In(CH3)3, used to prepare certain semiconducting materials.[34][35]
History[edit]
In 1863, the German chemists Ferdinand Reich and Hieronymous Theodor Richter were testing ores from the mines around Freiberg, Saxony. They dissolved the minerals pyrite, arsenopyrite, galena and sphalerite in hydrochloric acid and distilled raw zinc chloride. Reich, who was color-blind, employed Richter as an assistant for detecting the colored spectral lines. Knowing that ores from that region sometimes contain thallium, they searched for the green thallium emission spectrum lines. Instead, they found a bright blue line. Because that blue line did not match any known element, they hypothesized a new element was present in the minerals. They named the element indium, from the indigo color seen in its spectrum, after the Latin indicum, meaning ‘of India’.[36][37][38][39]
Richter went on to isolate the metal in 1864.[40] An ingot of 0.5 kg (1.1 lb) was presented at the World Fair 1867.[41] Reich and Richter later fell out when the latter claimed to be the sole discoverer.[39]
Occurrence[edit]

Indium is created by the long-lasting (up to thousands of years) s-process (slow neutron capture) in low-to-medium-mass stars (range in mass between 0.6 and 10 solar masses). When a silver-109 atom captures a neutron, it transmutes into silver-110, which then undergoes beta decay to become cadmium-110. Capturing further neutrons, it becomes cadmium-115, which decays to indium-115 by another beta decay. This explains why the radioactive isotope is more abundant than the stable one.[42] The stable indium isotope, indium-113, is one of the p-nuclei, the origin of which is not fully understood; although indium-113 is known to be made directly in the s- and r-processes (rapid neutron capture), and also as the daughter of very long-lived cadmium-113, which has a half-life of about eight quadrillion years, this cannot account for all indium-113.[43][44]
Indium is the 68th most abundant element in Earth’s crust at approximately 50 ppb. This is similar to the crustal abundance of silver, bismuth and mercury. It very rarely forms its own minerals, or occurs in elemental form. Fewer than 10 indium minerals such as roquesite (CuInS2) are known, and none occur at sufficient concentrations for economic extraction.[45] Instead, indium is usually a trace constituent of more common ore minerals, such as sphalerite and chalcopyrite.[46][47] From these, it can be extracted as a by-product during smelting.[48] While the enrichment of indium in these deposits is high relative to its crustal abundance, it is insufficient, at current prices, to support extraction of indium as the main product.[45]
Different estimates exist of the amounts of indium contained within the ores of other metals.[49][50] However, these amounts are not extractable without mining of the host materials (see Production and availability). Thus, the availability of indium is fundamentally determined by the rate at which these ores are extracted, and not their absolute amount. This is an aspect that is often forgotten in the current debate, e.g. by the Graedel group at Yale in their criticality assessments,[51] explaining the paradoxically low depletion times some studies cite.[52][48]
Production and availability[edit]

World production trend[53]
Indium is produced exclusively as a by-product during the processing of the ores of other metals. Its main source material are sulfidic zinc ores, where it is mostly hosted by sphalerite.[48] Minor amounts are probably also extracted from sulfidic copper ores. During the roast-leach-electrowinning process of zinc smelting, indium accumulates in the iron-rich residues. From these, it can be extracted in different ways. It may also be recovered directly from the process solutions. Further purification is done by electrolysis.[54] The exact process varies with the mode of operation of the smelter.[8][48]
Its by-product status means that indium production is constrained by the amount of sulfidic zinc (and copper) ores extracted each year. Therefore, its availability needs to be discussed in terms of supply potential. The supply potential of a by-product is defined as that amount which is economically extractable from its host materials per year under current market conditions (i.e. technology and price).[55] Reserves and resources are not relevant for by-products, since they cannot be extracted independently from the main-products.[48] Recent estimates put the supply potential of indium at a minimum of 1,300 t/yr from sulfidic zinc ores and 20 t/yr from sulfidic copper ores.[48] These figures are significantly greater than current production (655 t in 2016).[56] Thus, major future increases in the by-product production of indium will be possible without significant increases in production costs or price. The average indium price in 2016 was US$240/kg, down from US$705/kg in 2014.[57]
China is a leading producer of indium (290 tonnes in 2016), followed by South Korea (195 t), Japan (70 t) and Canada (65 t).[56] The Teck Resources refinery in Trail, British Columbia, is a large single-source indium producer, with an output of 32.5 tonnes in 2005, 41.8 tonnes in 2004 and 36.1 tonnes in 2003.
The primary consumption of indium worldwide is LCD production. Demand rose rapidly from the late 1990s to 2010 with the popularity of LCD computer monitors and television sets, which now account for 50% of indium consumption.[58] Increased manufacturing efficiency and recycling (especially in Japan) maintain a balance between demand and supply. According to the UNEP, indium’s end-of-life recycling rate is less than 1%.[59]
Applications[edit]

A magnified image of an LCD screen showing RGB pixels. Individual transistors are seen as white dots in the bottom part.
In 1924, indium was found to have a valued property of stabilizing non-ferrous metals, and that became the first significant use for the element.[60] The first large-scale application for indium was coating bearings in high-performance aircraft engines during World War II, to protect against damage and corrosion; this is no longer a major use of the element.[54] New uses were found in fusible alloys, solders, and electronics. In the 1950s, tiny beads of indium were used for the emitters and collectors of PNP alloy-junction transistors. In the middle and late 1980s, the development of indium phosphide semiconductors and indium tin oxide thin films for liquid-crystal displays (LCD) aroused much interest. By 1992, the thin-film application had become the largest end use.[61][62]
Indium(III) oxide and indium tin oxide (ITO) are used as a transparent conductive coating on glass substrates in electroluminescent panels.[63] Indium tin oxide is used as a light filter in low-pressure sodium-vapor lamps. The infrared radiation is reflected back into the lamp, which increases the temperature within the tube and improves the performance of the lamp.[62]
Indium has many semiconductor-related applications. Some indium compounds, such as indium antimonide and indium phosphide,[64] are semiconductors with useful properties: one precursor is usually trimethylindium (TMI), which is also used as the semiconductor dopant in II–VI compound semiconductors.[65] InAs and InSb are used for low-temperature transistors and InP for high-temperature transistors.[54] The compound semiconductors InGaN and InGaP are used in light-emitting diodes (LEDs) and laser diodes.[66] Indium is used in photovoltaics as the semiconductor copper indium gallium selenide (CIGS), also called CIGS solar cells, a type of second-generation thin-film solar cell.[67] Indium is used in PNP bipolar junction transistors with germanium: when soldered at low temperature, indium does not stress the germanium.[54]

A video on indium lung, an illness caused by indium exposure
Indium wire is used as a vacuum seal and a thermal conductor in cryogenics and ultra-high-vacuum applications, in such manufacturing applications as gaskets that deform to fill gaps.[68] Owing to its great plasticity and adhesion to metals, Indium sheets are sometimes used for cold-soldering in microwave circuits and waveguide joints, where direct soldering is complicated. Indium is an ingredient in the gallium–indium–tin alloy galinstan, which is liquid at room temperature and replaces mercury in some thermometers.[69] Other alloys of indium with bismuth, cadmium, lead, and tin, which have higher but still low melting points (between 50 and 100 °C), are used in fire sprinkler systems and heat regulators.[54]
Indium is one of many substitutes for mercury in alkaline batteries to prevent the zinc from corroding and releasing hydrogen gas.[70] Indium is added to some dental amalgam alloys to decrease the surface tension of the mercury and allow for less mercury and easier amalgamation.[71]
Indium’s high neutron-capture cross-section for thermal neutrons makes it suitable for use in control rods for nuclear reactors, typically in an alloy of 80% silver, 15% indium, and 5% cadmium.[72] In nuclear engineering, the (n,n’) reactions of 113In and 115In are used to determine magnitudes of neutron fluxes.[73]
In 2009, Professor Mas Subramanian and associates at Oregon State University discovered that indium can be combined with yttrium and manganese to form an intensely blue, non-toxic, inert, fade-resistant pigment, YInMn blue, the first new inorganic blue pigment discovered in 200 years.[74]
Biological role and precautions[edit]
| Hazards | |
|---|---|
| GHS labelling: | |
| Pictograms |  |
| Signal word | Warning |
| Hazard statements | H302, H312, H315, H319, H332, H335 |
| Precautionary statements | P261, P280, P305+P351+P338[75] |
| NFPA 704 (fire diamond) |
2 0 0 |
Indium has no metabolic role in any organism. In a similar way to aluminium salts, indium(III) ions can be toxic to the kidney when given by injection.[76] Indium tin oxide and indium phosphide harm the pulmonary and immune systems, predominantly through ionic indium,[77] though hydrated indium oxide is more than forty times as toxic when injected, measured by the quantity of indium introduced.[76] Radioactive indium-111 (in very small amounts on a chemical basis) is used in nuclear medicine tests, as a radiotracer to follow the movement of labeled proteins and white blood cells in the body.[78][79] Indium compounds are mostly not absorbed upon ingestion and are only moderately absorbed on inhalation; they tend to be stored temporarily in the muscles, skin, and bones before being excreted, and the biological half-life of indium is about two weeks in humans.[80]
People can be exposed to indium in the workplace by inhalation, ingestion, skin contact, and eye contact. Indium lung is a lung disease characterized by pulmonary alveolar proteinosis and pulmonary fibrosis, first described by Japanese researchers in 2003. As of 2010, 10 cases had been described, though more than 100 indium workers had documented respiratory abnormalities.[81] The National Institute for Occupational Safety and Health has set a recommended exposure limit (REL) of 0.1 mg/m3 over an eight-hour workday.[82]
See also[edit]
References[edit]
- ^ «Standard Atomic Weights: Indium». CIAAW. 2011.
- ^ Mangum, B. W. (1989). «Determination of the Indium Freezing-point and Triple-point Temperatures». Metrologia. 26 (4): 211. Bibcode:1989Metro..26..211M. doi:10.1088/0026-1394/26/4/001.
- ^ Unstable In(0) carbonyls and clusters have been detected, see [1], p. 6.
- ^ Guloy, A. M.; Corbett, J. D. (1996). «Synthesis, Structure, and Bonding of Two Lanthanum Indium Germanides with Novel Structures and Properties». Inorganic Chemistry. 35 (9): 2616–22. doi:10.1021/ic951378e. PMID 11666477.
- ^ Lide, D. R., ed. (2005). «Magnetic susceptibility of the elements and inorganic compounds». CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ W. M. Haynes (2010). David R. Lide (ed.). CRC Handbook of Chemistry and Physics: A Ready-reference Book of Chemical and Physical Data. CRC Press. ISBN 978-1-4398-2077-3.
- ^ a b c Alfantazi, A. M.; Moskalyk, R. R. (2003). «Processing of indium: a review». Minerals Engineering. 16 (8): 687–694. doi:10.1016/S0892-6875(03)00168-7.
- ^ Binder, Harry H. (1999). Lexicon der chemischen Elemente (in German). S. Hirzel Verlag. ISBN 978-3-7776-0736-8.
- ^ a b Dean, John A. (523). Lange’s handbook of chemistry (Fifteenth ed.). McGraw-Hill, Inc. ISBN 978-0-07-016190-0.
- ^ Greenwood and Earnshaw, p. 222
- ^ a b Greenwood and Earnshaw, p. 252
- ^ Okamoto, H. (2012). «Hg-In phase diagram». Journal of Phase Equilibria and Diffusion. 33 (2): 159–160. doi:10.1007/s11669-012-9993-3. S2CID 93043767.
- ^ Iliev, S. P.; Chen, X.; Pathan, M. V.; Tagarielli, V. L. (2017-01-23). «Measurements of the mechanical response of Indium and of its size dependence in bending and indentation». Materials Science and Engineering: A. 683: 244–251. doi:10.1016/j.msea.2016.12.017. hdl:10044/1/43082.
- ^ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). «Thallium». Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 892–893. ISBN 978-3-11-007511-3.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Greenwood and Earnshaw, p. 256
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 8.20. ISBN 1-4398-5511-0.
- ^ Greenwood and Earnshaw, p. 255
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), «The NUBASE evaluation of nuclear and decay properties», Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729….3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ Dvornický, R.; Šimkovic, F. (13–16 June 2011). «Second unique forbidden β decay of 115In and neutrino mass». AIP Conf. Proc. AIP Conference Proceedings. 1417 (33): 33. Bibcode:2011AIPC.1417…33D. doi:10.1063/1.3671032.
- ^ «IUPAC Periodic Table of the Isotopes» (PDF). ciaaw.org. IUPAC. 1 October 2013. Retrieved 21 June 2016.
- ^ a b Anthony John Downs (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. ISBN 978-0-7514-0103-5.
- ^ Greenwood and Earnshaw, p. 286
- ^ Greenwood and Earnshaw, pp. 263–7
- ^ Greenwood and Earnshaw, p. 288
- ^ Greenwood and Earnshaw, pp. 270–1
- ^ a b Sinclair, Ian; Worrall, Ian J. (1982). «Neutral complexes of the indium dihalides». Canadian Journal of Chemistry. 60 (6): 695–698. doi:10.1139/v82-102.
- ^ Greenwood and Earnshaw, p. 287
- ^ Beck, Horst Philipp; Wilhelm, Doris (1991). «In7Cl9—A New»Old» Compound in the System In-Cl». Angewandte Chemie International Edition in English. 30 (7): 824–825. doi:10.1002/anie.199108241.
- ^ Dronskowski, Richard (1995). «Synthesis, Structure, and Decay of In4Br7». Angewandte Chemie International Edition in English. 34 (10): 1126–1128. doi:10.1002/anie.199511261.
- ^ Fischer, E. O.; Hofmann, H. P. (1957). «Metall-cyclopentadienyle des Indiums». Angewandte Chemie (in German). 69 (20): 639–640. Bibcode:1957AngCh..69..639F. doi:10.1002/ange.19570692008.
- ^ Beachley O. T.; Pazik J. C.; Glassman T. E.; Churchill M. R.; Fettinger J.C.; Blom R. (1988). «Synthesis, characterization and structural studies of In(C5H4Me) by x-ray diffraction and electron diffraction techniques and a reinvestigation of the crystalline state of In(C5H5) by x-ray diffraction studies». Organometallics. 7 (5): 1051–1059. doi:10.1021/om00095a007.
- ^ Shenai, Deo V.; Timmons, Michael L.; Dicarlo, Ronald L.; Lemnah, Gregory K.; Stennick, Robert S. (2003). «Correlation of vapor pressure equation and film properties with trimethylindium purity for the MOVPE grown III–V compounds». Journal of Crystal Growth. 248: 91–98. Bibcode:2003JCrGr.248…91S. doi:10.1016/S0022-0248(02)01854-7.
- ^ Shenai, Deodatta V.; Timmons, Michael L.; Dicarlo, Ronald L.; Marsman, Charles J. (2004). «Correlation of film properties and reduced impurity concentrations in sources for III/V-MOVPE using high-purity trimethylindium and tertiarybutylphosphine». Journal of Crystal Growth. 272 (1–4): 603–608. Bibcode:2004JCrGr.272..603S. doi:10.1016/j.jcrysgro.2004.09.006.
- ^ Reich, F.; Richter, T. (1863). «Ueber das Indium». Journal für Praktische Chemie (in German). 90 (1): 172–176. doi:10.1002/prac.18630900122. S2CID 94381243.
- ^ Venetskii, S. (1971). «Indium». Metallurgist. 15 (2): 148–150. doi:10.1007/BF01088126.
- ^ Greenwood and Earnshaw, p. 244
- ^ a b Weeks, Mary Elvira (1932). «The Discovery of the Elements: XIII. Some Spectroscopic Studies». Journal of Chemical Education. 9 (8): 1413–1434. Bibcode:1932JChEd…9.1413W. doi:10.1021/ed009p1413.[permanent dead link]
- ^ Reich, F.; Richter, T. (1864). «Ueber das Indium». Journal für Praktische Chemie (in German). 92 (1): 480–485. doi:10.1002/prac.18640920180.
- ^ Schwarz-Schampera, Ulrich; Herzig, Peter M. (2002). Indium: Geology, Mineralogy, and Economics. Springer. ISBN 978-3-540-43135-0.
- ^ Boothroyd, A. I. (2006). «Heavy elements in stars». Science. 314 (5806): 1690–1691. doi:10.1126/science.1136842. PMID 17170281. S2CID 116938510.
- ^ Arlandini, C.; Käppeler, F.; Wisshak, K.; Gallino, R.; Lugaro, M.; Busso, M.; Straniero, O. (1999). «Neutron Capture in Low-Mass Asymptotic Giant Branch Stars: Cross Sections and Abundance Signatures». The Astrophysical Journal. 525 (2): 886–900. arXiv:astro-ph/9906266. Bibcode:1999ApJ…525..886A. doi:10.1086/307938. S2CID 10847307.
- ^ Zs; Käppeler, F.; Theis, C.; Belgya, T.; Yates, S. W. (1994). «Nucleosynthesis in the Cd-In-Sn region». The Astrophysical Journal. 426: 357–365. Bibcode:1994ApJ…426..357N. doi:10.1086/174071.
- ^ a b Frenzel, Max (2016). «The distribution of gallium, germanium and indium in conventional and non-conventional resources — Implications for global availability (PDF Download Available)». ResearchGate. doi:10.13140/rg.2.2.20956.18564. Retrieved 2017-06-02.
- ^ Frenzel, Max; Hirsch, Tamino; Gutzmer, Jens (July 2016). «Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type — A meta-analysis». Ore Geology Reviews. 76: 52–78. doi:10.1016/j.oregeorev.2015.12.017.
- ^ Bachmann, Kai; Frenzel, Max; Krause, Joachim; Gutzmer, Jens (June 2017). «Advanced Identification and Quantification of In-Bearing Minerals by Scanning Electron Microscope-Based Image Analysis». Microscopy and Microanalysis. 23 (3): 527–537. Bibcode:2017MiMic..23..527B. doi:10.1017/S1431927617000460. ISSN 1431-9276. PMID 28464970. S2CID 6751828.
- ^ a b c d e f Frenzel, Max; Mikolajczak, Claire; Reuter, Markus A.; Gutzmer, Jens (June 2017). «Quantifying the relative availability of high-tech by-product metals – The cases of gallium, germanium and indium». Resources Policy. 52: 327–335. doi:10.1016/j.resourpol.2017.04.008.
- ^ «Mineral Commodities Summary 2007: Indium» (PDF). United States Geological Survey. Retrieved 2007-12-26.
- ^ Werner, T. T.; Mudd, G. M.; Jowitt, S. M. (2015-10-02). «Indium: key issues in assessing mineral resources and long-term supply from recycling». Applied Earth Science. 124 (4): 213–226. doi:10.1179/1743275815Y.0000000007. ISSN 0371-7453. S2CID 128555024.
- ^ Graedel, T. E.; Barr, Rachel; Chandler, Chelsea; Chase, Thomas; Choi, Joanne; Christoffersen, Lee; Friedlander, Elizabeth; Henly, Claire; Jun, Christine (2012-01-17). «Methodology of Metal Criticality Determination». Environmental Science & Technology. 46 (2): 1063–1070. Bibcode:2012EnST…46.1063G. doi:10.1021/es203534z. ISSN 0013-936X. PMID 22191617.
- ^ Harper, E. M.; Kavlak, Goksin; Burmeister, Lara; Eckelman, Matthew J.; Erbis, Serkan; Sebastian Espinoza, Vicente; Nuss, Philip; Graedel, T. E. (2015-08-01). «Criticality of the Geological Zinc, Tin, and Lead Family». Journal of Industrial Ecology. 19 (4): 628–644. doi:10.1111/jiec.12213. ISSN 1530-9290. S2CID 153380535.[permanent dead link]
- ^ U.S. Geological Survey – Historical Statistics for Mineral and Material Commodities in the United States; INDIUM STATISTICS // USGS, April 1, 2014
- ^ a b c d e Greenwood and Earnshaw, p. 247
- ^ Frenzel, Max; Tolosana-Delgado, Raimon; Gutzmer, Jens (December 2015). «Assessing the supply potential of high-tech metals – A general method». Resources Policy. 46, Part 2: 45–58. doi:10.1016/j.resourpol.2015.08.002.
- ^ a b Indium — in: USGS Mineral Commodity Summaries (PDF). United States Geological Survey. 2017.
- ^ Kelly, TD; Matos, GR (2015). «Historical Statistics for Mineral and Material Commodities in the United States». Retrieved 2017-06-02.
- ^ «Indium Price Supported by LCD Demand and New Uses for the Metal». Geology.com. Archived from the original (PDF) on 2007-12-21. Retrieved 2007-12-26.
- ^ «USGS Mineral Commodity Summaries 2011» (PDF). USGS and USDI. Retrieved August 2, 2011.
- ^ French, Sidney J. (1934). «A story of indium». Journal of Chemical Education. 11 (5): 270. Bibcode:1934JChEd..11..270F. doi:10.1021/ed011p270.
- ^ Tolcin, Amy C. «Mineral Yearbook 2007: Indium» (PDF). United States Geological Survey.
- ^ a b Downs, Anthony John (1993). Chemistry of Aluminium, Gallium, Indium, and Thallium. Springer. pp. 89 and 106. ISBN 978-0-7514-0103-5.
- ^ «The Electroluminescent Light Sabre». Nanotechnology News Archive. Azonano. June 2, 2005. Archived from the original on October 12, 2007. Retrieved 2007-08-29.
- ^ Bachmann, K. J. (1981). «Properties, Preparation, and Device Applications of Indium Phosphide». Annual Review of Materials Science. 11: 441–484. Bibcode:1981AnRMS..11..441B. doi:10.1146/annurev.ms.11.080181.002301.
- ^ Shenai, Deodatta V.; Timmons, Michael L.; DiCarlo Jr., Ronald L.; Marsman, Charles J. (2004). «Correlation of film properties and reduced impurity concentrations in sources for III/V-MOVPE using high-purity trimethylindium and tertiarybutylphosphine». Journal of Crystal Growth. 272 (1–4): 603–608. Bibcode:2004JCrGr.272..603S. doi:10.1016/j.jcrysgro.2004.09.006.
- ^ Schubert, E. Fred (2003). Light-Emitting Diodes. Cambridge University Press. p. 16. ISBN 978-0-521-53351-5.
- ^ Powalla, M.; Dimmler, B. (2000). «Scaling up issues of CIGS solar cells». Thin Solid Films. 361–362 (1–2): 540–546. Bibcode:2000TSF…361..540P. doi:10.1016/S0040-6090(99)00849-4.
- ^ Weissler, G. L., ed. (1990). Vacuum physics and technology. San Diego: Acad. Press. p. 296. ISBN 978-0-12-475914-5.
- ^ Surmann, P; Zeyat, H (Nov 2005). «Voltammetric analysis using a self-renewable non-mercury electrode». Analytical and Bioanalytical Chemistry. 383 (6): 1009–13. doi:10.1007/s00216-005-0069-7. PMID 16228199. S2CID 22732411.
- ^ Geological Survey (U.S.) (2010). Minerals Yearbook, 2008, V. 1, Metals and Minerals. Government Printing Office. pp. 35–2. ISBN 978-1-4113-3015-3.
- ^ Powell L. V., Johnson G. H., Bales D. J. (1989). «Effect of Admixed Indium on Mercury Vapor Release from Dental Amalgam». Journal of Dental Research. 68 (8): 1231–3. CiteSeerX 10.1.1.576.2654. doi:10.1177/00220345890680080301. PMID 2632609. S2CID 28342583.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Scoullos, Michael J. (2001-12-31). «Other types of cadmium alloys». Mercury, cadmium, lead: handbook for sustainable heavy metals policy and regulation. p. 222. ISBN 978-1-4020-0224-3.
- ^ Berger, Harold; National Bureau Of Standards, United States; Committee E-7 On Nondestructive Testing, American Society for Testing and Materials (1976). «Image Detectors for Other Neutron Energies». Practical applications of neutron radiography and gaging: a symposium. pp. 50–51.
- ^ Kupferschmidt, Kai (2019-05-02). «In search of blue». Science. American Association for the Advancement of Science (AAAS). 364 (6439): 424–429. Bibcode:2019Sci…364..424K. doi:10.1126/science.364.6439.424. ISSN 0036-8075. PMID 31048474. S2CID 143434096.
- ^ «Indium 57083».
- ^ a b Castronovo, F. P.; Wagner, H. N. (October 1971). «Factors Affecting the Toxicity of the Element Indium». British Journal of Experimental Pathology. 52 (5): 543–559. PMC 2072430. PMID 5125268.
- ^ Gwinn, W. M.; Qu, W.; Bousquet, R. W.; Price, H.; Shines, C. J.; Taylor, G. J.; Waalkes, M. P.; Morgan, D. L. (2014). «Macrophage Solubilization and Cytotoxicity of Indium-Containing Particles as in vitro Correlates to Pulmonary Toxicity in vivo». Toxicological Sciences. 144 (1): 17–26. doi:10.1093/toxsci/kfu273. PMC 4349143. PMID 25527823.
- ^ «IN-111 FACT SHEET» (PDF). Nordion(Canada), Inc. Archived from the original (PDF) on 3 December 2011. Retrieved 23 September 2012.
- ^ Van Nostrand, D.; Abreu, S. H.; Callaghan, J. J.; Atkins, F. B.; Stoops, H. C.; Savory, C. G. (May 1988). «In-111-labeled white blood cell uptake in noninfected closed fracture in humans: prospective study». Radiology. 167 (2): 495–498. doi:10.1148/radiology.167.2.3357961. PMID 3357961.
- ^ Nordberg, Gunnar F.; Fowler, Bruce A.; Nordberg, Monica (7 August 2014). Handbook on the Toxicology of Metals (4th ed.). Academic Press. p. 845. ISBN 978-0-12-397339-9.
- ^ Sauler, Maor; Gulati, Mridu (December 2012). «Newly Recognized Occupational and Environmental Causes of Chronic Terminal Airways and Parenchymal Lung Disease». Clinics in Chest Medicine. 33 (4): 667–680. doi:10.1016/j.ccm.2012.09.002. PMC 3515663. PMID 23153608.
- ^ «CDC – NIOSH Pocket Guide to Chemical Hazards – Indium». www.cdc.gov. Retrieved 2015-11-06.
Sources[edit]
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
External links[edit]
- Indium at The Periodic Table of Videos (University of Nottingham)
- Reducing Agents > Indium low valent
- NIOSH Pocket Guide to Chemical Hazards (Centers for Disease Control and Prevention)
Индий в таблице менделеева занимает 49 место, в 5 периоде.
| Символ | In |
| Номер | 49 |
| Атомный вес | 114.8180000 |
| Латинское название | Indium |
| Русское название | Индий |
Как самостоятельно построить электронную конфигурацию? Ответ здесь
Электронная схема индия
In: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1
Короткая запись:
In: [Kr]5s2 4d10 5p1
Порядок заполнения оболочек атома индия (In) электронами:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d →
5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
На подуровне ‘s’ может находиться до 2 электронов, на ‘s’ — до 6, на
‘d’ — до 10 и на ‘f’ до 14
Индий имеет 49 электронов,
заполним электронные оболочки в описанном выше порядке:
2 электрона на 1s-подуровне
2 электрона на 2s-подуровне
6 электронов на 2p-подуровне
2 электрона на 3s-подуровне
6 электронов на 3p-подуровне
2 электрона на 4s-подуровне
10 электронов на 3d-подуровне
6 электронов на 4p-подуровне
2 электрона на 5s-подуровне
10 электронов на 4d-подуровне
1 электрон на 5p-подуровне
Степень окисления индия
Атомы индия в соединениях имеют степени окисления 3, 2, 1.
Степень окисления — это условный заряд атома в соединении: связь в молекуле
между атомами основана на разделении электронов, таким образом, если у атома виртуально увеличивается
заряд, то степень окисления отрицательная (электроны несут отрицательный заряд), если заряд уменьшается,
то степень окисления положительная.
Ионы индия
Валентность In
Атомы индия в соединениях проявляют валентность III, II, I.
Валентность индия характеризует способность атома In к образованию хмических связей.
Валентность следует из строения электронной оболочки атома, электроны, участвующие в образовании
химических соединений называются валентными электронами. Более обширное определение валентности это:
Число химических связей, которыми данный атом соединён с другими атомами
Валентность не имеет знака.
Квантовые числа In
Квантовые числа определяются последним электроном в конфигурации,
для атома In эти числа имеют значение N = 5, L = 1, Ml = -1, Ms = +½
Видео заполнения электронной конфигурации (gif):
Результат:

Энергия ионизации
Чем ближе электрон к центру атома — тем больше энергии необходимо, что бы его оторвать.
Энергия, затрачиваемая на отрыв электрона от атома называется энергией ионизации и обозначается Eo.
Если не указано иное, то энергия ионизации — это энергия отрыва первого электрона, также существуют энергии
ионизации для каждого последующего электрона.
Энергия ионизации In:
Eo = 558 кДж/моль
— Что такое ион читайте в статье.
Перейти к другим элементам таблицы менделеева
Где In в таблице менделеева?
Таблица Менделеева
Скачать таблицу менделеева в хорошем качестве
| |||
| Внешний вид простого вещества | |||
|---|---|---|---|
|
| |||
| Свойства атома | |||
| Имя, символ, номер | И́ндий / Indium (In), 49 | ||
| Атомная масса (молярная масса) | 114,818 а. е. м. (г/моль) | ||
| Электронная конфигурация | [Kr] 4d10 5s2 5p1 | ||
| Радиус атома | 166 пм | ||
| Химические свойства | |||
| Ковалентный радиус | 144 пм | ||
| Радиус иона | (+3e) 81 пм | ||
| Электроотрицательность | 1,78 (шкала Полинга) | ||
| Электродный потенциал | −0,34 В | ||
| Степени окисления | 3 | ||
| Энергия ионизации (первый электрон) | 558,0 (5,78) кДж/моль (эВ) | ||
| Термодинамические свойства простого вещества | |||
| Плотность (при н. у.) | 7,31 г/см³ | ||
| Температура плавления | 429.32 K (156,17°C) | ||
| Температура кипения | 2353 K (2079,85°C) | ||
| Теплота плавления | 3,24 кДж/моль | ||
| Теплота испарения | 225,1 кДж/моль | ||
| Молярная теплоёмкость | 26,7[1] Дж/(K·моль) | ||
| Молярный объём | 15,7 см³/моль | ||
| Кристаллическая решётка простого вещества | |||
| Структура решётки | Тетрагональная | ||
| Параметры решётки | a=3,252 c=4,946[2] Å | ||
| Отношение c/a | 1,52 | ||
| Температура Дебая | 129 K(-144.15°C) | ||
| Прочие характеристики | |||
| Теплопроводность | (300 K) 81,8 Вт/(м·К) |
И́ндий — элемент главной подгруппы третьей группы пятого периода периодической системы химических элементов Д. И. Менделеева, атомный номер 49. Обозначается символом In (лат. Indium). Относится к группе лёгких металлов. Простое вещество индий (CAS-номер: 7440-74-6) — ковкий, легкоплавкий, очень мягкий металл серебристо-белого цвета. Сходен по химическим свойствам с алюминием и галлием, по внешнему виду с цинком.
Содержание
- 1 История
- 2 Происхождение названия
- 3 Геохимия и минералогия
- 4 Химические свойства
- 5 Физические свойства
- 5.1 Термодинамические параметры
- 5.2 Дополнительная информация
- 6 Получение
- 7 Применение
- 8 Биологическая роль
- 9 Изотопы
- 10 Примечания
- 11 Ссылки
История
Индий обнаружили немецкие химики Фердинанд Райх и Теодор Рихтер (Theodore Richter) в 1863 году при спектроскопическом исследовании цинковой обманки[3][4]. Они искали таллий, однако вместо зелёной линии этого элемента нашли в спектрах яркую неизвестную линию голубого цвета (Профессор Ф. Райх страдал дальтонизмом и не мог различать цвета спектральных линий, поэтому все наблюдения регистрировал его ассистент Рихтер)[5]. Впоследствии металл был выделен Рихтером в незначительном количестве, но на Всемирной выставке 1867 г. уже был представлен полукилограммовый слиток индия[6].
Происхождение названия
Яркая эмиссионная линия в спектре индия — цвета индиго.
Геохимия и минералогия
Учитывая электронную структуру атома индия, он относится к халькофильным элементам (18 электронов в предпоследнем слое). В настоящее время известно менее 10 индиевых минералов: самородный индий, рокезит CuInS2, индит FeIn2S4, кадмоиндит (CdIn2S4), джалиндит In(OH)3, сакуранит (CuZnFe)3InS4 и патрукит (Cu,Fe,Zn)2(Sn,In)S4[7]. В основном индий находится в виде изоморфной примеси в раннем высокожелезистом сфалерите, где его содержание достигает десятых долей процента. В некоторых разновидностях халькопирита и станнина содержание индия составляет сотые-десятые процента, а в касситерите и пирротине — тысячные доли процента. В пирите, арсенопирите, вольфрамите и некоторых других минералах концентрация индия граммы на тонну. Промышленное значение для получения металла пока имеют сфалерит и другие минералы, содержащие не менее 0,1 % индия. Индий самостоятельных месторождений не образует, а входит в состав руд месторождений других металлов. Наиболее высокое содержание индия установлено в рудах касситеритоносных скарнов и сульфидно-касситеритовых месторождений различных типов. Содержание индия в земной коре (кларк) 0,25 г/т (он в три раза более распространён, чем серебро), в морской воде 0,018 мг/л[8].
Химические свойства
- Электроотрицательность 1,78.
- Устойчив и не тускнеет в сухом воздухе при комнатной температуре, но выше 800 °C горит фиолетово-синим пламенем с образованием оксида.
- Растворяется в серной и соляной кислотах, быстрее — в азотной и хлорной, с плавиковой кислотой медленно реагирует при нагревании, органические кислоты (муравьиная, уксусная, щавелевая, лимонная) постепенно растворяют индий.
- С растворами щелочей, даже кипящими, заметно не реагирует.
- Реагирует с хлором и бромом.
- При нагревании реагирует с иодом, серой (выше 620 °C), селеном, теллуром, диоксидом серы (выше 600 °C), парами фосфора.
- Степень окисления от +1 до +3, наиболее устойчивы 3-валентные соединения.
Физические свойства
- Критическая температура сверхпроводимости (атмосферное давление, массивные образцы) 3,405 К
- Плотность: 7,362 (20 °C, г/см³)
- 7,023 (157 °C, г/см³)
- 5,763 (2109 °C, г/см³)
- Давление паров (в мм рт. ст.):
- 0,01 (912 °C)
- 0,1 (1042 °C)
- 1 (1205 °C)
- 10 (1414 °C)
- 100 (1688 °C)
- Удельная теплоемкость при постоянном давлении (0-150 °C): 0,238 Дж/г·K
Термодинамические параметры
- Стандартная энтальпия образования ΔH (298 К): 0 кДж/моль (т)
- Стандартная энергия Гиббса образования ΔG (298 К): 0 кДж/моль (т)
- Стандартная энтропия образования S (298 К): 57,82 Дж/моль·K (т)
- Стандартная мольная теплоемкость Cp (298 К): 26,74 Дж/моль·K (т)
- Энтальпия плавления ΔHпл: 3,26 кДж/моль
- Энтальпия кипения ΔHкип: 227,6 кДж/моль
Дополнительная информация
- Сплав с 40 % платины имеет золотисто-жёлтый цвет. Известно «зеленое золото» — сплав 75 % золота с 20 % серебра и 5 % индия[9].
- Твёрдость по Бринеллю 9 МПа, по Моосу 1,2.
- Водород малорастворим в металлическом индии — менее 1 мл на 100 г индия.
Получение
Получают из отходов и промежуточных продуктов производства цинка, свинца и олова. Это сырьё содержит от 0,001 % до 0,1 % индия. Из исходного сырья производят концентрат индия, из концентрата — черновой металл, который затем рафинируют. Исходное сырьё обрабатывают серной кислотой и переводят индий в раствор, из которого гидролитическим осаждением выделяют концентрат. Из концентрата черновой металл извлекают цементацией на цинке и алюминии. Для рафинирования используются различные методы, например зонная плавка.
Стоимость индия в 2006 году составила около 700$ за кг.
Применение
- Используется в микроэлектронике как акцепторная примесь к германию и кремнию.
- Применяется в производстве жидкокристаллических экранов для нанесения прозрачных плёночных электродов из оксида индия-олова.
- Компонент ряда легкоплавких припоев и сплавов (так, жидкий при комнатной температуре галинстан содержит 21,5 % индия). Обладает высокой адгезией ко многим материалам, позволяя спаивать, например, металл со стеклом.
- Иногда применяется (чистый или в сплаве с серебром) для покрытия зеркал, в частности, автомобильных фар, при этом отражающая способность зеркал не хуже, чем у серебряных, а стойкость к воздействию атмосферы (особенно сероводорода) — больше. В покрытии астрономических зеркал используется постоянство коэффициента отражения индия в видимой части спектра.
- Материал для фотоэлементов.
- Соединения используются как люминофоры.
- Покрытие юбок алюминиевых поршней дизельных двигателей для снижения износа.
- Арсенид индия применяется как высокотемпературный термоэлектрический материал с очень высокой эффективностью, для увеличения эффективности обычно легируется 10 % фосфида индия.
- Изотопы индия 111In и 113mIn используются в качестве радиофармацевтических препаратов.
- Точка плавления индия (429,7485 К или 156,5985 °C) — одна из определяющих точек международной температурной шкалы ITS-90.
- Индий входит в состав «голубого золота»[10].
- Электрохимическая система индий-оксид ртути служит для создания чрезвычайно стабильных во времени источников тока (аккумуляторов) высокой удельной энергоёмкости для специальных целей.
- Ортофосфат индия используется в качестве добавки к зубным цементам[11].
- В технике высокого вакуума индий используется в качестве уплотнителя (прокладки, покрытия); в частности, при герметизации космических аппаратов и мощных ускорителей элементарных частиц.
- Индий имеет высокое сечение захвата тепловых нейтронов и может быть использован для управления атомным реактором, хотя более удобно применение его соединений в комбинации с другими элементами, хорошо захватывающими нейтроны. Так, оксид индия находит применение в атомной технике для изготовления стекла, применяемого для поглощения тепловых нейтронов. Наиболее широко распространённый состав такого стекла — оксид бора (33 %), оксид кадмия (55 %), оксид индия (12 %).
В последние годы мировое потребление индия быстро растёт и в 2005 достигло 850 тонн.
Биологическая роль
Индий не обнаружен в составе каких-либо жизненно важных соединений. Тем не менее, утверждается[кем?], что препараты индия могут стимулировать метаболизм.
Изотопы
Природный индий состоит из двух изотопов — стабильного 113In (изотопная распространённость 4,29 %) и бета-радиоактивного 115In (95,71 %; период полураспада 4,41·1014 лет).
Примечания
- ↑ Редкол.:Кнунянц И. Л. (гл. ред.) Химическая энциклопедия: в 5 т. — Москва: Советская энциклопедия, 1990. — Т. 2. — С. 226. — 671 с. — 100 000 экз.
- ↑ WebElements Periodic Table of the Elements | Indium | crystal structures
- ↑ Reich, F.; Richter, T. (1863). «Ueber das Indium». Journal für Praktische Chemie 90 (1): 172–176. DOI:10.1002/prac.18630900122.
- ↑ Reich, F.; Richter, T. (1864). «Ueber das Indium». Journal für Praktische Chemie 92 (1): 480–485. DOI:10.1002/prac.18640920180.
- ↑ Кафедра физической и коллоидной химии Южного федерального университета
- ↑ Schwarz-Schampera Ulrich Indium: Geology, Mineralogy, and Economics. — Springer, 2002. — ISBN 9783540431350
- ↑ ИНДИЙ | Энциклопедия Кругосвет
- ↑ J.P. Riley and Skirrow G. Chemical Oceanography V. I, 1965.
- ↑ С. И. Венецкий. О редких и рассеянных. Рассказы о металлах. М.: «Металлургия», 1980. [1]
- ↑ Голубое золото
- ↑ XuMuK.ru — ИНДИЙ — Химическая энциклопедия
Ссылки
| Индий на Викискладе? |
- Индий на Webelements
- Индий в Популярной библиотеке химических элементов
- Индий в chemport.ru
| Периодическая система химических элементов Д. И. Менделеева | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo |
|
| |
|---|
| Eu, Sm, Li, Cs, Rb, K, Ra, Ba, Sr, Ca, Na, Ac, La, Ce, Pr, Nd, Pm, Gd, Tb, Mg, Y, Dy, Am, Ho, Er, Tm, Lu, Sc, Pu, Th, Np, U, Hf, Be, Al, Ti, Zr, Yb, Mn, V, Nb, Pa, Cr, Zn, Ga, Fe, Cd, In, Tl, Co, Ni, Te, Mo, Sn, Pb, H2, W, Sb, Bi, Ge, Re, Cu, Tc, Te, Rh, Po, Hg, Ag, Pd, Os, Ir, Pt, Au Элементы расположены в порядке возрастания стандартного электродного потенциала. |
| Соединения индия |
|---|
| Антимонид индия (InSb) • Арсенид индия (InAs) • Бромид индия(I) (InBr) • Бромид индия(II) (InBr2) • Бромид индия(III) (InBr3) • Гидрид индия (InH3) • Гидроксид индия(III) (In(OH)3) • Гидросульфат индия (InHSO4) • Дихлор-алюминат индия (In[AlH4]Cl2) • Иодид индия(II) (InI2) • Иодид индия(I) (InI) • Иодид индия(III) (InI3) • Нитрат индия(III) (In(NO3)3) • Нитрид индия(III) (InN) • Оксид индия(I) (In2O) • Оксид индия(II) (InO) • Оксид индия(III) (In2O3) • Станнид индия (InSn) • Сульфат индия(III) (In2(SO4)3) • Сульфид индия(I) (In2S) • Сульфид индия(II) (InS) • Сульфид индия(III) (In2S3) • Триметилиндий (In(CH3)3) • Трипропилиндий (In(C3H7)3) • Трифенилиндий (In(C6H5)3) • Триэтилиндий (In(C2H5)3) • Фосфид индия (InP) • Фторид индия(III) (InF3) • Хлорид индия(I) (InCl) • Хлорид индия(II) (InCl2) Хлорид индия(III) (InCl3) |
|
|